Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1. What is the average value of the rate constantcalculated from the three data sets? Express the rate constant in the provided units to threesignificant
1. What is the average value of the rate constantcalculated from the three data sets?
Express the rate constant in the provided units to threesignificant figures.
2. What is the rate of disappearance ofNO when [NO]=0.0910 and [O2]=0.0244?
Express the rate in molarity per second to three significantfigures.
3. What is the rate of disappearanceof O2 when [NO]=0.0910and [O2]=0.0244?
Express the rate in molarity per second to three significantfigures.
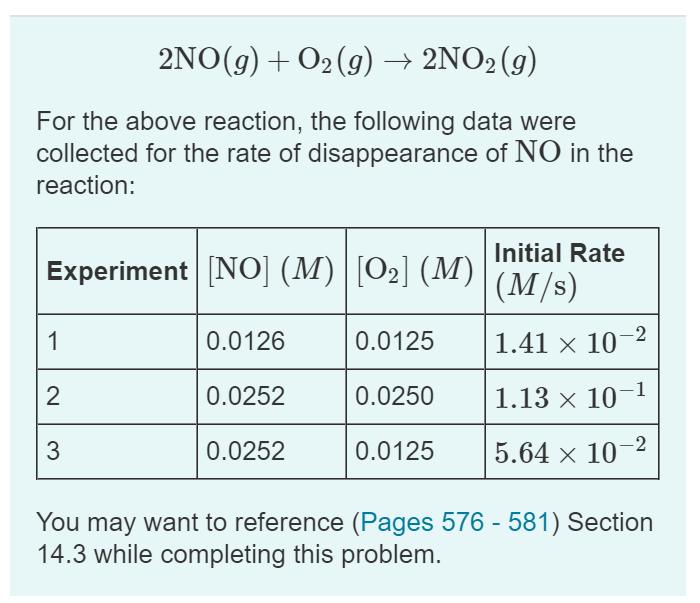
2NO(g) + O2(g) 2NO2 (g) For the above reaction, the following data were collected for the rate of disappearance of NO in the reaction: Experiment [NO] (M)|[0] (M) 1 2 3 0.0126 0.0252 0.0252 0.0125 0.0250 0.0125 Initial Rate (M/s) 1.41 10-2 1.13 10-1 5.64 10- You may want to reference (Pages 576 - 581) Section 14.3 while completing this problem.
Step by Step Solution
★★★★★
3.54 Rating (157 Votes )
There are 3 Steps involved in it
Step: 1
For the this Now 2 NO2 g 0 8 Lets assume the rate law is Rate k NO 0 B From the given data we g...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


