Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1- What is the purpose of this experiment? (Electrophilic Aromatic Substitution: Synthesis of Iodosalicylamide). 2- Calculations the Theoretical yield of this experiment? Background As you


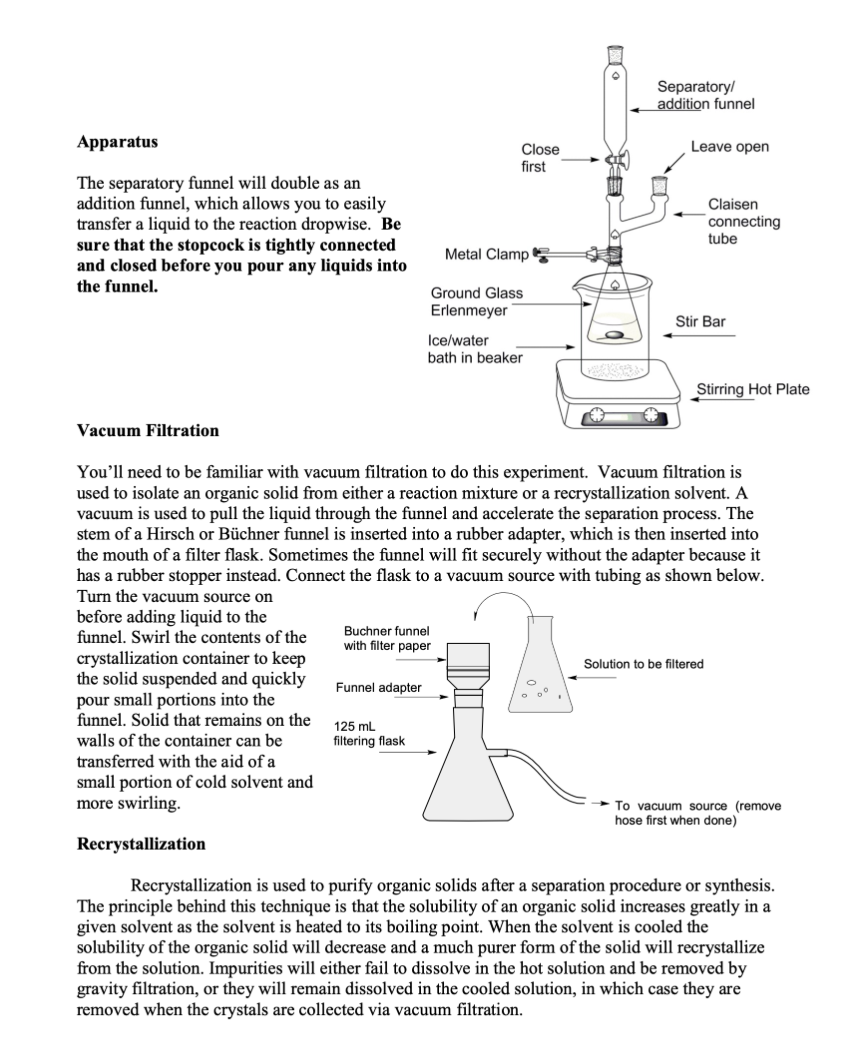

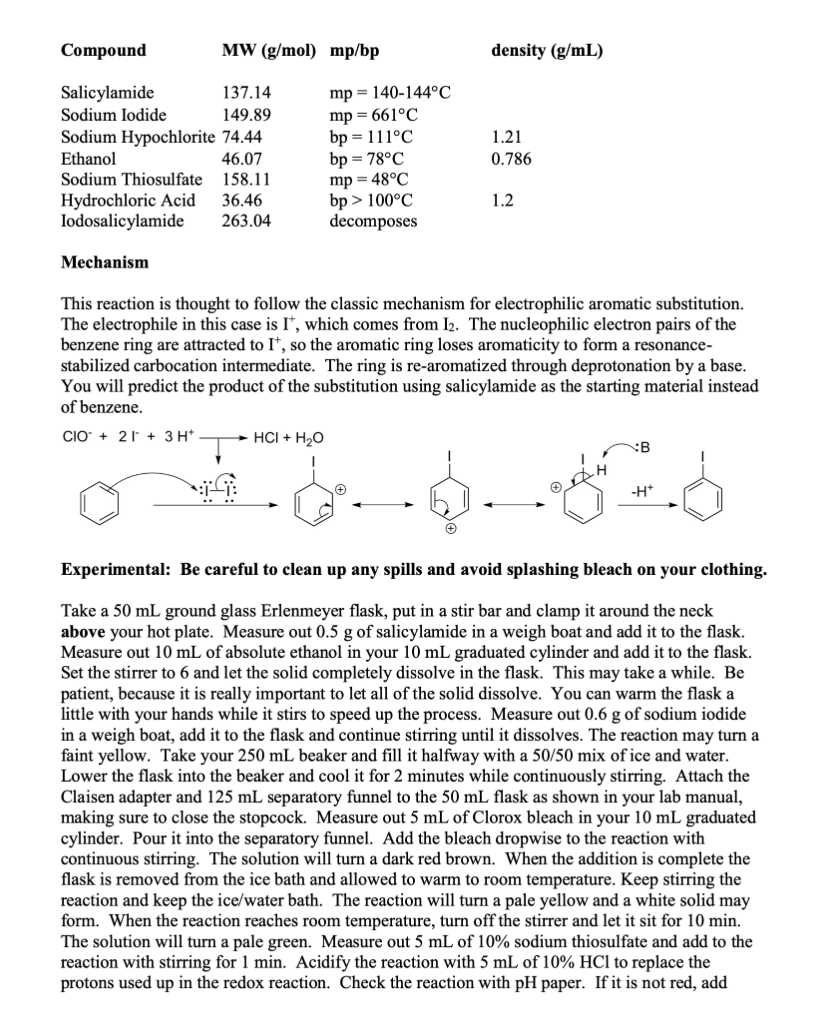
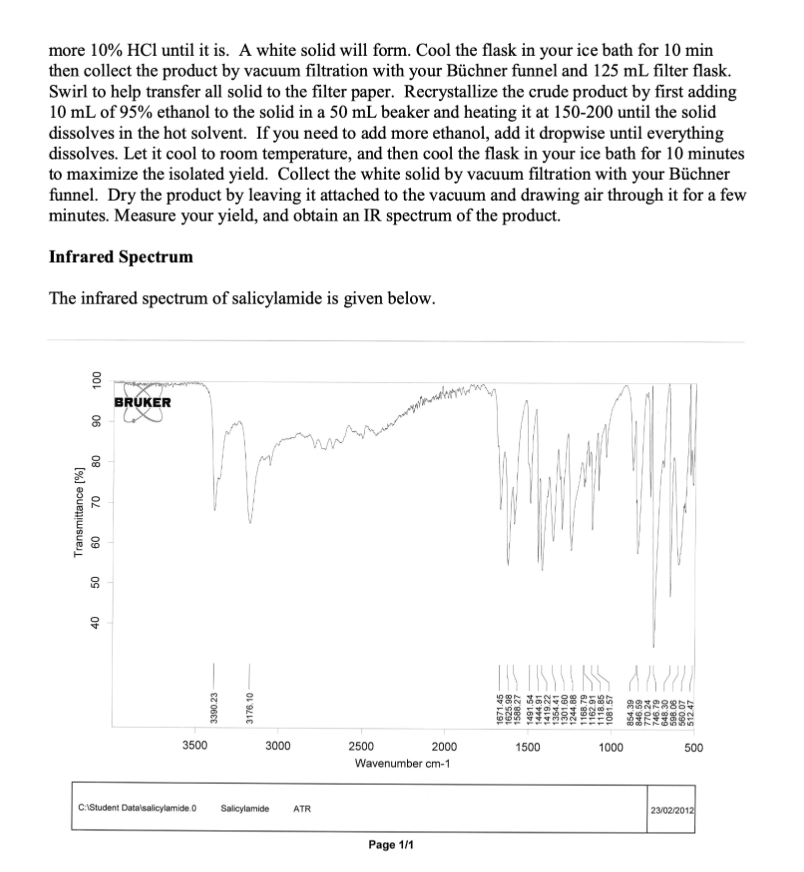
1- What is the purpose of this experiment? (Electrophilic Aromatic Substitution: Synthesis of Iodosalicylamide).
2- Calculations the Theoretical yield of this experiment?
Background As you have no doubt discussed in Chemistry 302, or will learn shortly, benzene and other aromatic compounds, though they contain pi bonds like alkenes and alkynes, react very differently. Alkenes and alkynes undergo addition reactions readily and the pi bonds "disappear" to make new sigma bonds. Benzene and its derivatives, on the other hand, undergo a reaction known as electrophilic aromatic substitution (EAS). Substitution implies by nature that something is replacing something else in the starting material. The reactions that fit in this category are numerous and both biological and laboratory versions exist. One very interesting example of EAS in life is the biosynthesis of the thyroid hormone thyroxine (shown below). Tyrosine is first iodinated using an enzyme called an iodoperoxidase, which generates the electrophilic species I+. This is an EAS reaction. You will use a laboratory method to generate I2, which will be used as a source of I+in this lab. Of course, enzymes are capable of helping to place reactive species exactly where they need to be for a reaction to achieve a specific outcome. In thyroxine, every possible ortho position relative to the hydroxyl group is iodinated. You will perform a similar analysis of a different substrate and attempt to predict where the iodine will end up. Eventually in the biosynthesis, the two iodinated tyrosines are connected through an ether linkage to produce thyroxine. Thyroxine itself is one of a group of interconnected thyroid hormones whose varied functions include regulation of metabolic enzyme activities, so it is quite important in medicine. Methodology As you will eventually learn in Chemistry 302 , specific groups on a benzene ring have an effect on where incoming groups in an EAS reaction will end up. If a group makes the reaction faster than compared to just benzene, the group is described as an activating group. If the group makes the reaction slower than compared to just benzene, the group is described as a deactivating group. Activators are able to donate electron density to the aromatic ring because they usually possess a lone pair of electrons that are conjugated to the aromatic ring. Deactivators withdraw electron density, as they usually have a partial positive or full positive formal charge. The strangest example of deactivators are the halogens, I,Br, and Cl. When these are on a benzene ring, their electronegativity results in electron density lost from the ring, even though they have lone pairs. Furthermore, the same groups can be categorized as ortho/para directors or meta directors. Your organic chemistry textbook will have a much more detailed list, but there are some easy ways to predict which effect a group has. Ortho/para directors usually have an atom connected to the ring that has at least one lone pair of electrons. A good example is aniline, which has an NH2 group with a lone pair attached to the benzene ring. All activating groups are ortho/para directors. Meta directors, on the other hand, have an atom directly connected to the ring that is multiply bonded to a more electronegative atom. The nitro (NO2) group has a double bond to one of the oxygens, which is more electronegative than nitrogen. Meta directors are always deactivating groups. Take a look at salicylamide below and consider how you would categorize the OH and the amide. Once both effects are known, then the task becomes predicting where the incoming electrophile (I+)will end up on the ring. You should end up with two potential products. Your task is to perform the reaction and assign the regiochemistry of the product using IR spectroscopy. To do that, you'll need to delve into the depths of the "fingerprint region," something very few organic chemistry students are brave enough to do! The Fingerprint Region - What is its use? Since NMR became the analytical tool of choice for organic chemists, IR is used less to characterize the structures of organic compounds. However, it can still be very useful. One of the ways it can help is in the determination of the substitution pattern for aromatic rings as seen in the table below. It should be relatively straightforward to determine the substitution pattern of your starting material, and then for your expected product. Use the IR spectral data of your product to confirm that you have made the expected product or a different one. Each of these molecular motions refers to a CH bend in the aromatic ring, called the " CH oop." In the CH Apparatus The separatory funnel will double as an addition funnel, which allows you to easily transfer a liquid to the reaction dropwise. Be sure that the stopcock is tightly connected and closed before you pour any liquids into the funnel. Vacuum Filtration You'll need to be familiar with vacuum filtration to do this experiment. Vacuum filtration is used to isolate an organic solid from either a reaction mixture or a recrystallization solvent. A vacuum is used to pull the liquid through the funnel and accelerate the separation process. The stem of a Hirsch or Bchner funnel is inserted into a rubber adapter, which is then inserted into the mouth of a filter flask. Sometimes the funnel will fit securely without the adapter because it has a rubber stopper instead. Connect the flask to a vacuum source with tubing as shown below. Turn the vacuum source on before adding liquid to the funnel. Swirl the contents of the crystallization container to keep the solid suspended and quickly pour small portions into the funnel. Solid that remains on the walls of the container can be transferred with the aid of a small portion of cold solvent and more swirling. Recrystallization Recrystallization is used to purify organic solids after a separation procedure or synthesis. The principle behind this technique is that the solubility of an organic solid increases greatly in a given solvent as the solvent is heated to its boiling point. When the solvent is cooled the solubility of the organic solid will decrease and a much purer form of the solid will recrystallize from the solution. Impurities will either fail to dissolve in the hot solution and be removed by gravity filtration, or they will remain dissolved in the cooled solution, in which case they are removed when the crystals are collected via vacuum filtration. The choice of recrystallization solvent is not trivial. The solvent must not react with the solid being dissolved and the solid must be significantly more soluble in the boiling solvent than in the cold solvent. The boiling point of the solvent should be high enough to take advantage of the compound's temperature coefficient of solubility and the freezing point should be low enough so it does not restrict the minimum temperature that can be used during cooling. Finally, impurities should be either insoluble in the hot solution or more soluble than the compound being purified. The rate of crystallization plays an important role in the purity of the final product. When the hot solution is gravity filtered, heat is lost to the glassware, quickly cooling the solution and causing rapid crystallization. Rapid crystallization results in small crystals, which readily absorb impurities on their surfaces. Conversely, large crystals, the product of slow cooling, may trap impurities within the crystal lattice. Cooling the solution to room temperature on the benchtop, then in an ice bath, should yield medium-sized crystals, which are usually the purest. It is important to realize that the solubility of the organic compound in the solvent decreases as the solution is cooled but it does not become zero. The very nature of recrystallization results in product being lost to the cold solvent. Addition of excess solvent will result in increased loss of product, so when appropriate, calculate how much solvent to use from the weight of your sample and its solubility. It is impossible to recover all of the material with which you began. The volume of solvent needed to dissolve your solid is usually given to you in Chem 304L but once you start work in a lab it's up to you to figure it out. Add the amount of solvent to the organic solid and heat to a boil. Add more solvent in small portions if necessary until the solid is dissolved. It is important that you do not add too large an excess of solvent as the compound is still soluble in the cold solvent but to a lesser extent than the boiling solvent. Do not let the solution boil for an extended amount of time as that will reduce the volume which can cause crystallization from a saturated solution. Once the solid is dissolved, place the container on the benchtop and allow it to cool to room temperature. Once at room temperature, place the container in an ice bath for ten minutes to ensure complete crystallization. Vacuum filter the white crystals using your Bchner or Hirsch funnel to collect them. Reaction and Properties This experiment involves three redox reactions. In the first, iodide ion and hypochlorite react in the presence of three protons to form iodine, which is the source of I+. This leaves some Ibehind, which combines with unused I2 to form I3. I3 - is reduced to water soluble Iby thiosulfate ion, 1. Nal/NaOCl/CH3CH2OH 2. Na2S2O3,HCl which stops the reaction. The line from the benzene ring connecting to the I means that you do not know its exact location on the ring. ClO+4I+3H+I2+II3+2S2O32HCl+H2O+2I2(red)I3(yellow/green)3I+O3SSSSO3 Mechanism This reaction is thought to follow the classic mechanism for electrophilic aromatic substitution. The electrophile in this case is I+, which comes from I2. The nucleophilic electron pairs of the benzene ring are attracted to I+, so the aromatic ring loses aromaticity to form a resonancestabilized carbocation intermediate. The ring is re-aromatized through deprotonation by a base. You will predict the product of the substitution using salicylamide as the starting material instead of benzene. Experimental: Be careful to clean up any spills and avoid splashing bleach on your clothing. Take a 50mL ground glass Erlenmeyer flask, put in a stir bar and clamp it around the neck above your hot plate. Measure out 0.5g of salicylamide in a weigh boat and add it to the flask. Measure out 10mL of absolute ethanol in your 10mL graduated cylinder and add it to the flask. Set the stirrer to 6 and let the solid completely dissolve in the flask. This may take a while. Be patient, because it is really important to let all of the solid dissolve. You can warm the flask a little with your hands while it stirs to speed up the process. Measure out 0.6g of sodium iodide in a weigh boat, add it to the flask and continue stirring until it dissolves. The reaction may turn a faint yellow. Take your 250mL beaker and fill it halfway with a 50/50 mix of ice and water. Lower the flask into the beaker and cool it for 2 minutes while continuously stirring. Attach the Claisen adapter and 125mL separatory funnel to the 50mL flask as shown in your lab manual, making sure to close the stopcock. Measure out 5mL of Clorox bleach in your 10mL graduated cylinder. Pour it into the separatory funnel. Add the bleach dropwise to the reaction with continuous stirring. The solution will turn a dark red brown. When the addition is complete the flask is removed from the ice bath and allowed to warm to room temperature. Keep stirring the reaction and keep the ice/water bath. The reaction will turn a pale yellow and a white solid may form. When the reaction reaches room temperature, turn off the stirrer and let it sit for 10min. The solution will turn a pale green. Measure out 5mL of 10% sodium thiosulfate and add to the reaction with stirring for 1min. Acidify the reaction with 5mL of 10%HCl to replace the protons used up in the redox reaction. Check the reaction with pH paper. If it is not red, add more 10%HCl until it is. A white solid will form. Cool the flask in your ice bath for 10min then collect the product by vacuum filtration with your Bchner funnel and 125mL filter flask. Swirl to help transfer all solid to the filter paper. Recrystallize the crude product by first adding 10mL of 95% ethanol to the solid in a 50mL beaker and heating it at 150200 until the solid dissolves in the hot solvent. If you need to add more ethanol, add it dropwise until everything dissolves. Let it cool to room temperature, and then cool the flask in your ice bath for 10 minutes to maximize the isolated yield. Collect the white solid by vacuum filtration with your Bchner funnel. Dry the product by leaving it attached to the vacuum and drawing air through it for a few minutes. Measure your yield, and obtain an IR spectrum of the product. Infrared SpectrumStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


