Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1.) Write a balanced chemical equation based on the following description: aqueous iron(III) chloride reacts with aqueous ammonium sulfide to make aqueous ammonium chloride
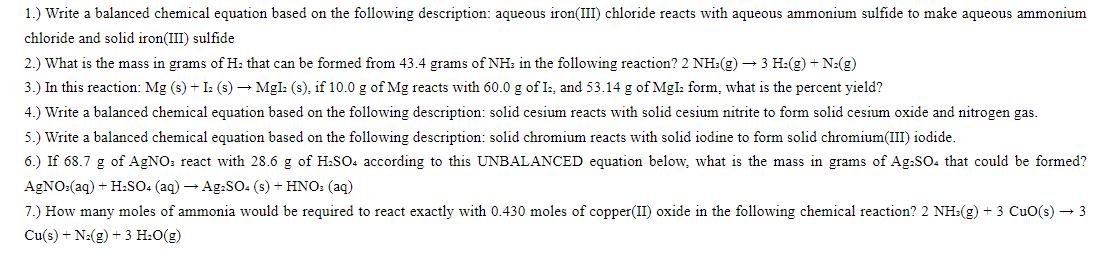
1.) Write a balanced chemical equation based on the following description: aqueous iron(III) chloride reacts with aqueous ammonium sulfide to make aqueous ammonium chloride and solid iron(III) sulfide 2.) What is the mass in grams of H: that can be formed from 43.4 grams of NH3 in the following reaction? 2 NH3(g) 3 H(g) + N(g) 3.) In this reaction: Mg (s) + I: (s) MgI: (s), if 10.0 g of Mg reacts with 60.0 g of I, and 53.14 g of Mgl: form, what is the percent yield? 4.) Write a balanced chemical equation based on the following description: solid cesium reacts with solid cesium nitrite to form solid cesium oxide and nitrogen gas. 5.) Write a balanced chemical equation based on the following description: solid chromium reacts with solid iodine to form solid chromium(III) iodide. 6.) If 68.7 g of AgNO: react with 28.6 g of HSO4 according to this UNBALANCED equation below, what is the mass in grams of Ag-SO. that could be formed? AgNO3(aq) + HSO4 (aq) Ag2SO4 (s) + HNO3(aq) 7.) How many moles of ammonia would be required to react exactly with 0.430 moles of copper(II) oxide in the following chemical reaction? 2 NH3(g) + 3 CuO(s) 3 Cu(s) + N(g) + 3 HO(g)
Step by Step Solution
★★★★★
3.37 Rating (172 Votes )
There are 3 Steps involved in it
Step: 1
1 FeCl3 aq NH4HS aq NH4Cl aq FeS s B al ance t he equation 2 F eCl3 aq 3 NH4 HS aq 2 NH4Cl aq Fe 2S3 ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


