Answered step by step
Verified Expert Solution
Question
1 Approved Answer
10. A solution contains 5 grams of glucose per 100 milliliters. Each mole of glucose weighs 180 grams. How many moles are there in
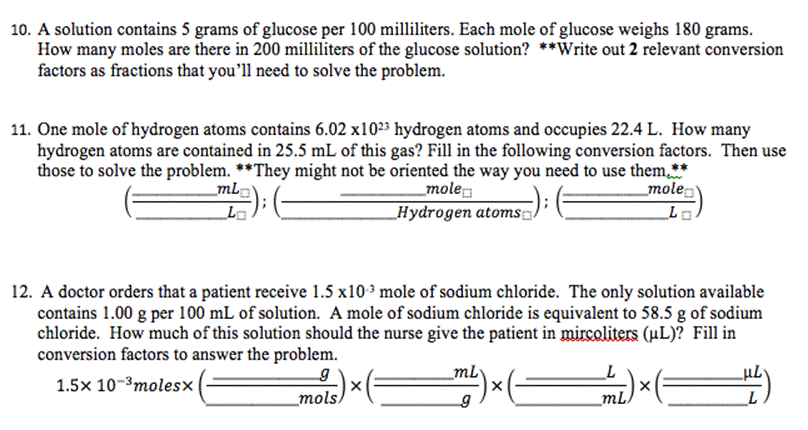
10. A solution contains 5 grams of glucose per 100 milliliters. Each mole of glucose weighs 180 grams. How many moles are there in 200 milliliters of the glucose solution? **Write out 2 relevant conversion factors as fractions that you'll need to solve the problem. 11. One mole of hydrogen atoms contains 6.02 x1023 hydrogen atoms and occupies 22.4 L. How many hydrogen atoms are contained in 25.5 mL of this gas? Fill in the following conversion factors. Then use those to solve the problem. **They might not be oriented the way you need to use them.** mole mL mole L Hydrogen atoms 12. A doctor orders that a patient receive 1.5 x10 mole of sodium chloride. The only solution available contains 1.00 g per 100 mL of solution. A mole of sodium chloride is equivalent to 58.5 g of sodium chloride. How much of this solution should the nurse give the patient in mircoliters (L)? Fill in conversion factors to answer the problem. g 1.5x 10-3 molesx _mols X mL 9 X L mL
Step by Step Solution
★★★★★
3.38 Rating (167 Votes )
There are 3 Steps involved in it
Step: 1
10 Given Solution contains 5 g glucose 100 mL Given 1 mol glucose 180 g glucose To find Moles of glu...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


