Question
10 g of aluminum at 392 F and 20 g of copper at an unknown temperature are dropped into 50 cm of ethyl alcohol
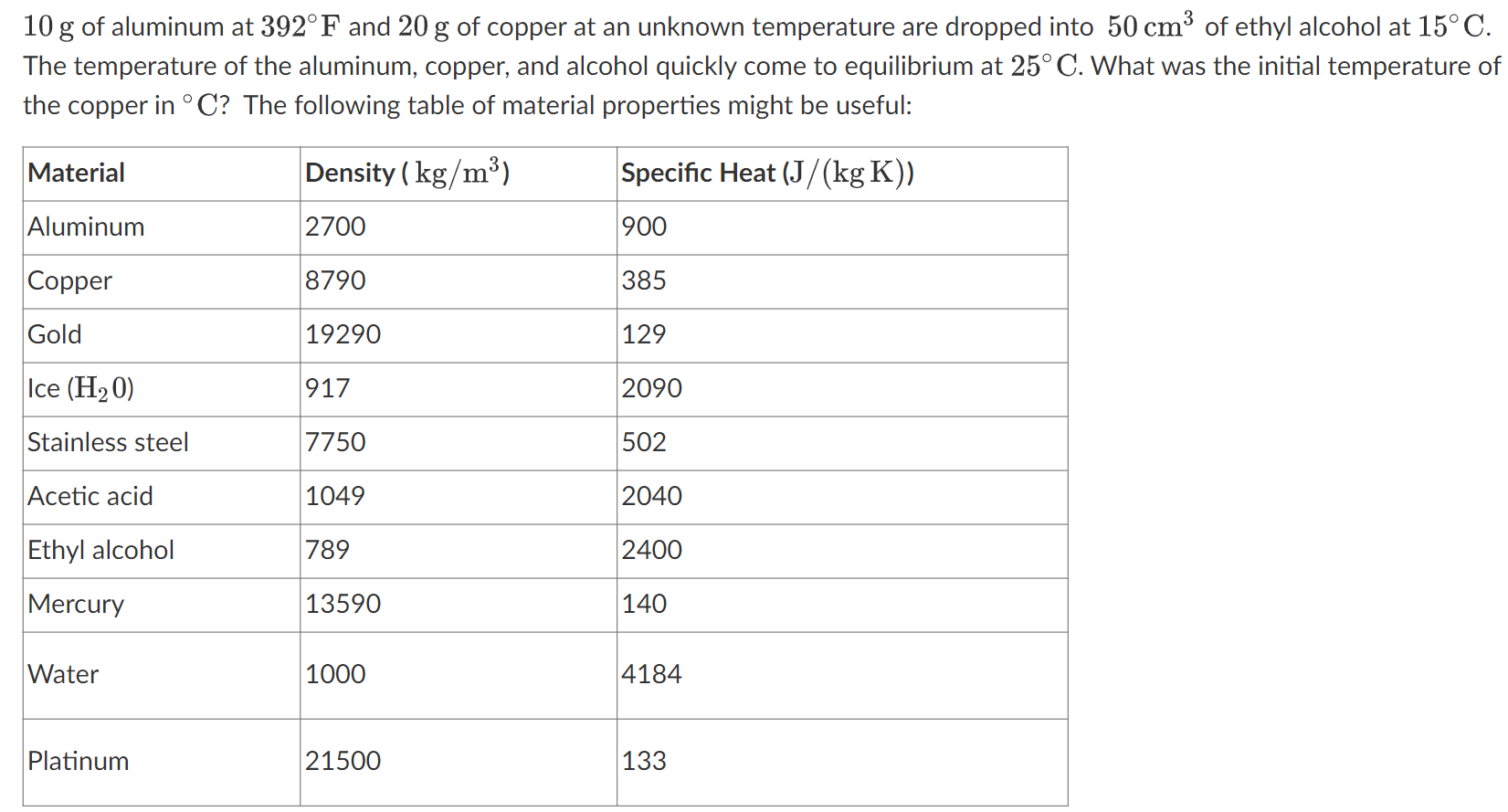
10 g of aluminum at 392 F and 20 g of copper at an unknown temperature are dropped into 50 cm of ethyl alcohol at 15 C. The temperature of the aluminum, copper, and alcohol quickly come to equilibrium at 25 C. What was the initial temperature of the copper in C? The following table of material properties might be useful: Material Density (kg/m) Specific Heat (J/(kg K)) Aluminum 2700 900 Copper 8790 385 Gold 19290 129 Ice (H20) 917 2090 Stainless steel 7750 502 Acetic acid 1049 2040 Ethyl alcohol 789 2400 Mercury 13590 140 Water 1000 4184 Platinum 21500 133
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Physics for Scientists and Engineers A Strategic Approach with Modern Physics
Authors: Randall D. Knight
4th edition
978-0134092508, 134092503, 133942651, 978-0133942651
Students also viewed these Physics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



