Answered step by step
Verified Expert Solution
Question
1 Approved Answer
10 help use sig fig to answer bottom questions The reaction of H, (g) + 2 ICI (g) > 1, (g) + 2 HCl (g)
10 help use sig fig to answer bottom questions 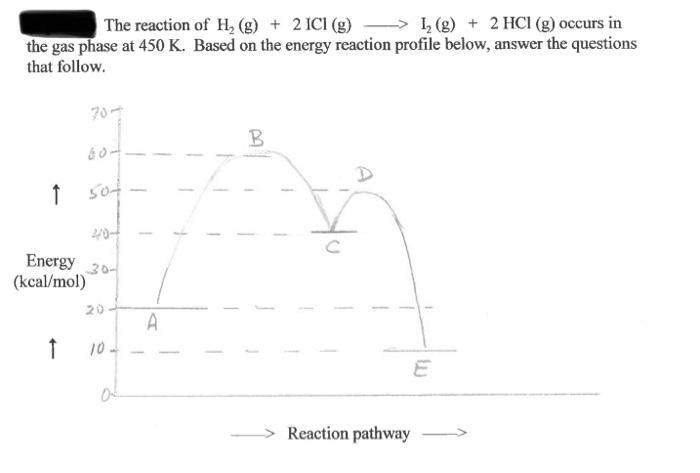

The reaction of H, (g) + 2 ICI (g) > 1, (g) + 2 HCl (g) occurs in the gas phase at 450 K. Based on the energy reaction profile below, answer the questions that follow. 70- B A 1 sot 20- Energy 30- (kcal/mol) 20 A 1 10- of Reaction pathway a) How many steps are there in the reaction mechanism? b) Which step is the rate-determining step? c) The reaction intermediate He is formed in this process. Where along the energy profile would it be found? (Use a letter or letters). d) is the formation of the products exothermic or endothermie? c) Using us the energy profile above, determine E, in kcal/mol for the slow step f) Using /usr the energy profile above, calculate AH in kcal/mol for the overall reaction g) Increasing the temperature will circle one): 1) decrease E ii) increase EA il) increase the value of the rule constant iv) decrease the value of the rate constant k v) alter the reaction mechanism h) The use of an effective catalyst in this process will circle one): i) increase the collision frequency ii) alter the reaction mechanism iii) increase the activation energy iv) decrease the value of the rate constant 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


