Question
Some equilibria relevant to blood buffering are shown below: H2CO3 H3PO4 H* + HCO3 pK = 6.4 H- H,PO, pK, = 6.8 pK =
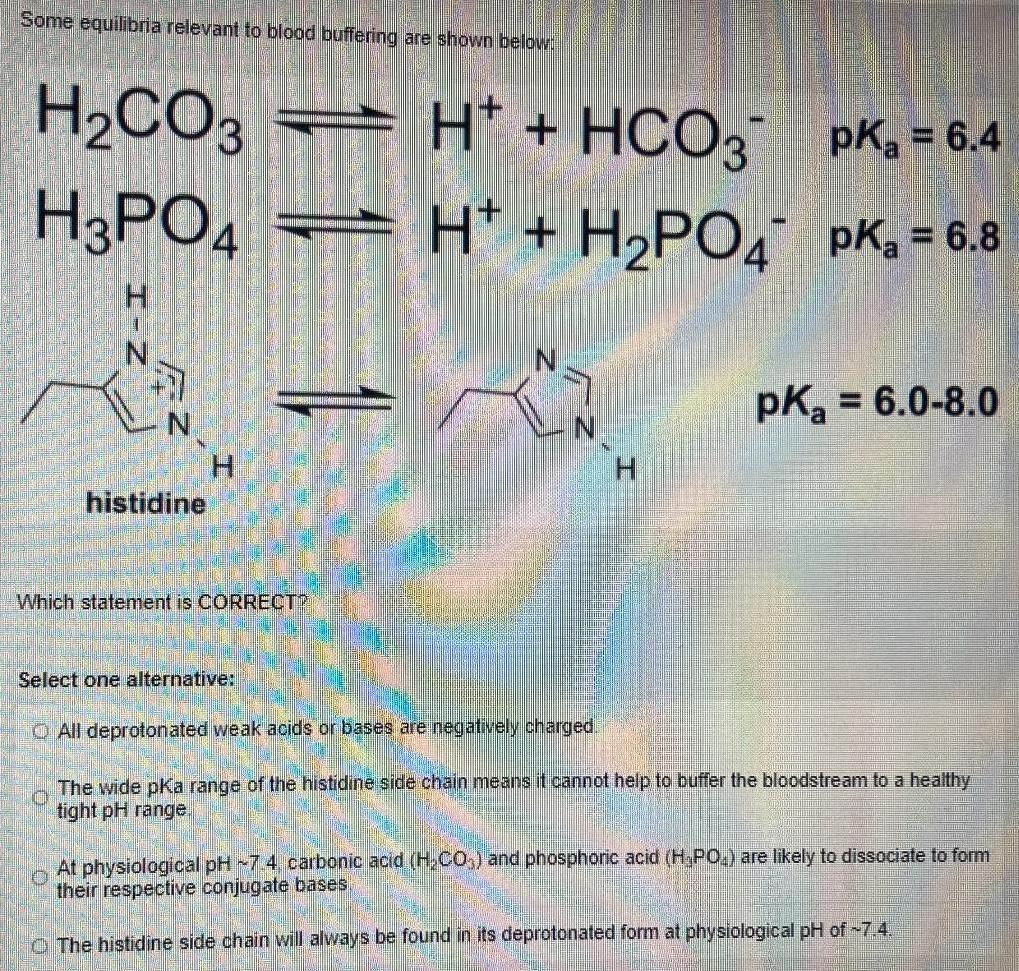
Some equilibria relevant to blood buffering are shown below: H2CO3 H3PO4 H* + HCO3 pK = 6.4 H- H,PO, pK, = 6.8 pK = 6.0-8.0 H. histidine Which statement is CORRECT? Select one alternative: O All deprotonated weak acids or bases are negatively charged. The wide pKa range of the histidine side chain means it cannot help to buffer the bloodstream to a healthy tight pH range At physiological pH 7 4 carbonic acid (H,CO,) and phosphoric acid (H,PO) are likely to dissociate to form their respective conjugate bases O The histidine side chain will always be found in its deprotonated form at physiological pH of -7.4.
Step by Step Solution
3.43 Rating (150 Votes )
There are 3 Steps involved in it
Step: 1
The third statement is correct ie At physiological pH 74 carbonic acid ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Microbiology A Systems Approach
Authors: Marjorie Kelly Cowan
4th edition
978-007340243, 73402435, 978-0073402437
Students also viewed these Biology questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



