Answered step by step
Verified Expert Solution
Question
1 Approved Answer
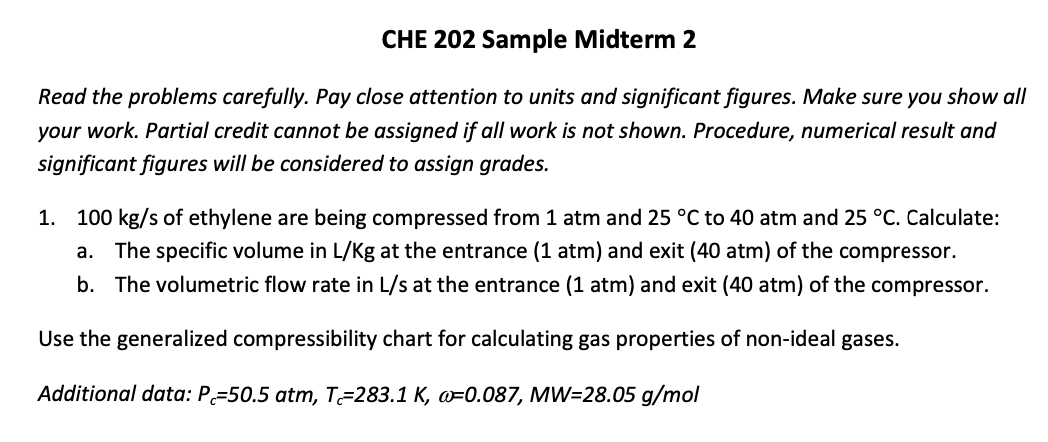
100 kg/s of ethylene are being compressed from 1 atm and 25 C to 40 atm and 25 C. Calculate: a. The specific volume in
100 kg/s of ethylene are being compressed from 1 atm and 25 C to 40 atm and 25 C. Calculate: a. The specific volume in L/Kg at the entrance (1 atm) and exit (40 atm) of the compressor. b. The volumetric flow rate in L/s at the entrance (1 atm) and exit (40 atm) of the compressor. Use the generalized compressibility chart for calculating the gas properties of non-ideal gases. Additional data: Pc=50.5 atm, Tc=283.1 K, =0.087, MW=28.05 g/mol
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


