Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1000 kg/h of a 30 wt% acetic acid-in-water solution is to be extracted at 20 C and 1 atm in a continuous countercurrent system with
1000 kg/h of a 30 wt% acetic acid-in-water solution is to be extracted at 20 C and 1 atm in a continuous countercurrent system with pure isopropyl ether to obtain a raffinate containing 10 wt% acetone. The solvent required is 1.5 times the minimum. The mixing point for minimum solvent is (xA,M = 13% , xD,M = 30%). Find out the solvent required for this system. Use the equilibrium miscibility data and tie line data
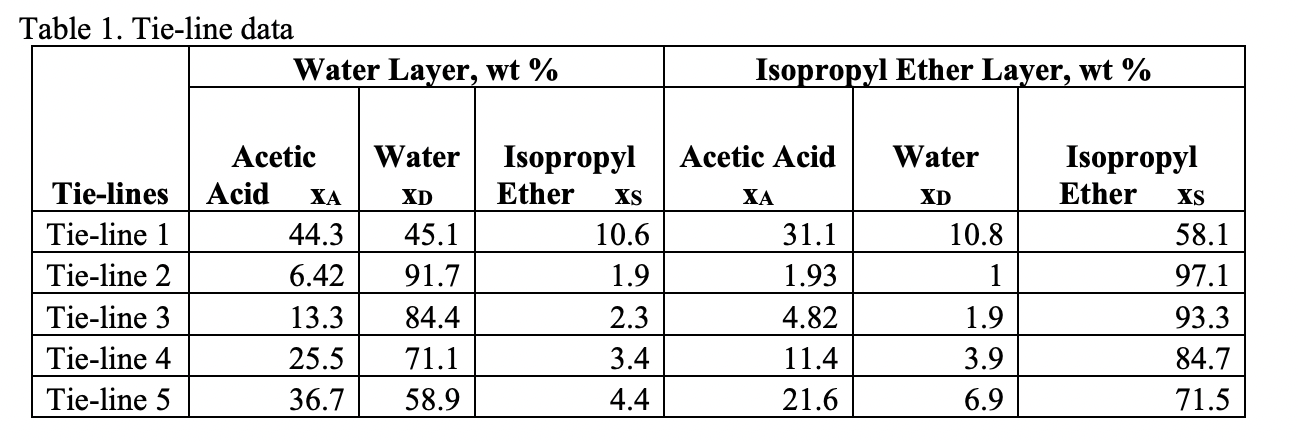
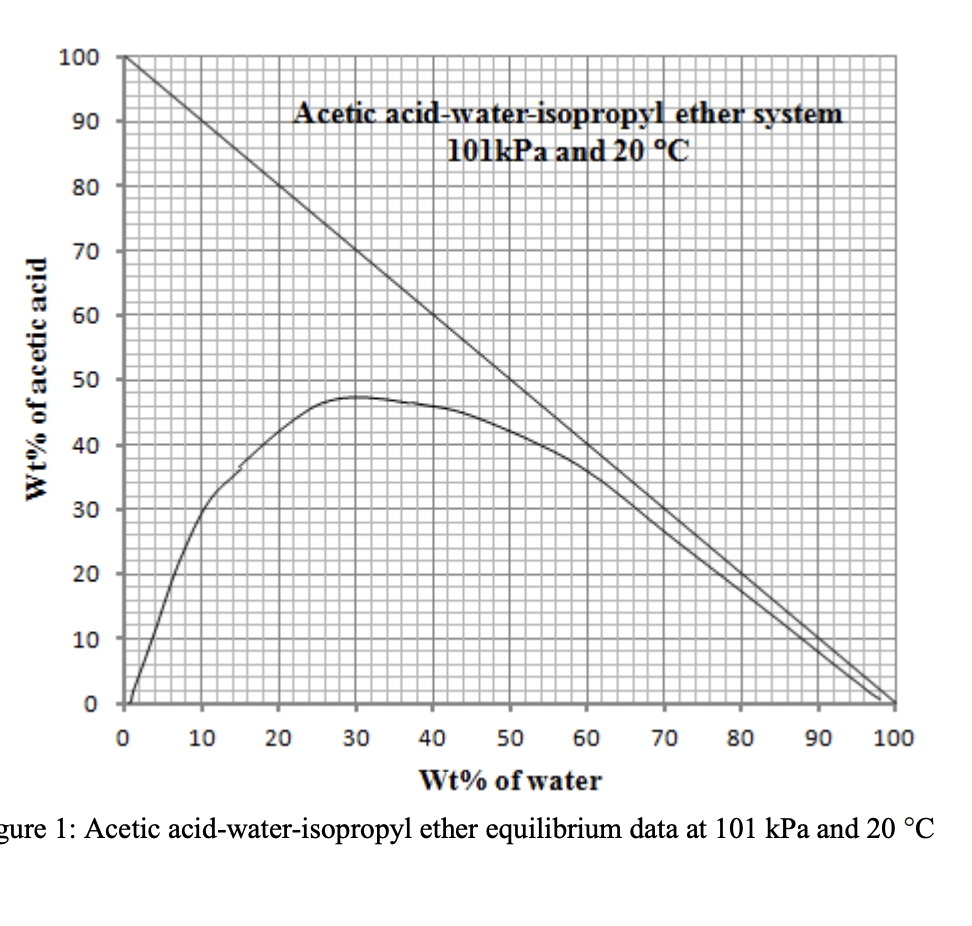 T-1.1-1 TR: 1:+11 ure 1: Acetic acid-water-isopropyl ether equilibrium data at 101kPa and 20C T-1.1-1 TR: 1:+11 ure 1: Acetic acid-water-isopropyl ether equilibrium data at 101kPa and 20C
T-1.1-1 TR: 1:+11 ure 1: Acetic acid-water-isopropyl ether equilibrium data at 101kPa and 20C T-1.1-1 TR: 1:+11 ure 1: Acetic acid-water-isopropyl ether equilibrium data at 101kPa and 20C Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


