Answered step by step
Verified Expert Solution
Question
1 Approved Answer
11. Combustion of 1 mole of C6H6(l) inside a closed container of constant volume liberates 900 kJ of heat energy. What would be the
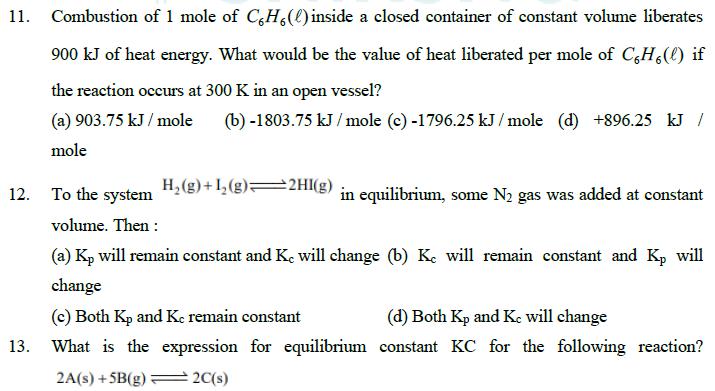
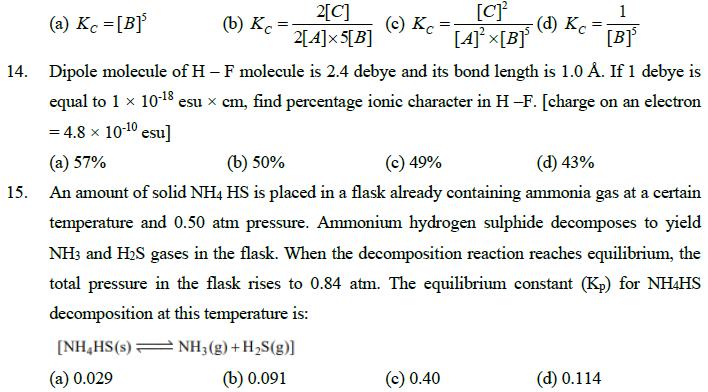
11. Combustion of 1 mole of C6H6(l) inside a closed container of constant volume liberates 900 kJ of heat energy. What would be the value of heat liberated per mole of C6H6(l) if the reaction occurs at 300 K in an open vessel? (a) 903.75 kJ/mole (b) -1803.75 kJ/mole (c) -1796.25 kJ/mole (d) +896.25 kJ / mole H2(g)+12(g) 2HI(g) 12. To the system in equilibrium, some N2 gas was added at constant volume. Then: 13. (a) Kp will remain constant and Ke will change (b) Ke will remain constant and Kp will change (c) Both Kp and Ke remain constant (d) Both Kp and Kc will change What is the expression for equilibrium constant KC for the following reaction? 2A(s)+5B(g) 2C(s) (a) Kc =[B] 2[C] (b) Kc (c) Kc = 2[A]5[B] [C] [A][B] 1 (d) Kc = [] 14. Dipole molecule of H - F molecule is 2.4 debye and its bond length is 1.0 . If 1 debye is equal to 1 10-18 esu x cm, find percentage ionic character in H-F. [charge on an electron = 4.8 10-10 esu] (a) 57% (b) 50% (c) 49% (d) 43% 15. An amount of solid NH4 HS is placed in a flask already containing ammonia gas at a certain temperature and 0.50 atm pressure. Ammonium hydrogen sulphide decomposes to yield NH3 and H2S gases in the flask. When the decomposition reaction reaches equilibrium, the total pressure in the flask rises to 0.84 atm. The equilibrium constant (Kp) for NH4HS decomposition at this temperature is: [NH HS(s) (a) 0.029 NH3(g) + H2S(g)] (b) 0.091 (c) 0.40 (d) 0.114
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


