Question
11. Dry the precipitate in the oven for about 30 minutes at 120C and determine the mass of the precipitate (subtract the weight of
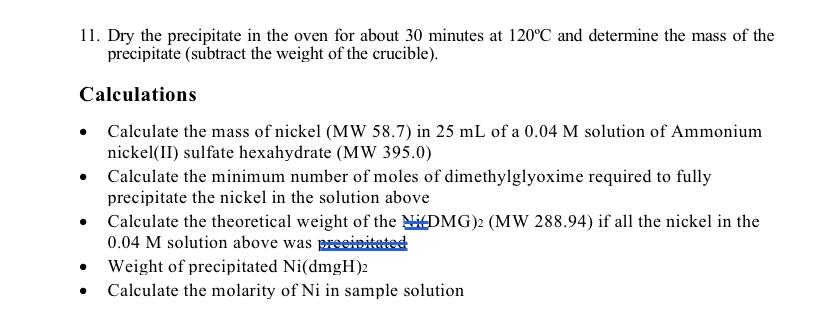
11. Dry the precipitate in the oven for about 30 minutes at 120C and determine the mass of the precipitate (subtract the weight of the crucible). Calculations Calculate the mass of nickel (MW 58.7) in 25 mL of a 0.04 M solution of Ammonium nickel(II) sulfate hexahydrate (MW 395.0) Calculate the minimum number of moles of dimethylglyoxime required to fully precipitate the nickel in the solution above Calculate the theoretical weight of the NDMG)2 (MW 288.94) if all the nickel in the 0.04 M solution above was precipitate . Weight of precipitated Ni(dmgH)2 Calculate the molarity of Ni in sample solution
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Applied Regression Analysis And Other Multivariable Methods
Authors: David G. Kleinbaum, Lawrence L. Kupper, Azhar Nizam, Eli S. Rosenberg
5th Edition
1285051084, 978-1285963754, 128596375X, 978-1285051086
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



