Question
8. One pound of an ideal gas undergoes an isentropic process from 95.3 psig and a volume of 0.6 ft to a final volume
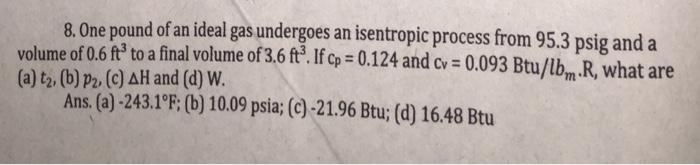
8. One pound of an ideal gas undergoes an isentropic process from 95.3 psig and a volume of 0.6 ft to a final volume of 3.6 ft. If cp = 0.124 and cv = 0.093 Btu/lb, R, what are (a) t2, (b) P2. (c) AH and (d) W. Ans. (a)-243.1 F; (b) 10.09 psia; (c) -21.96 Btu; (d) 16.48 Btu %3D %3D
Step by Step Solution
3.48 Rating (155 Votes )
There are 3 Steps involved in it
Step: 1
One pound of an ideal gas undergoes an isenbropic proc...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemical Principles
Authors: Steven S. Zumdahl, Donald J. DeCoste
7th edition
9781133109235, 1111580650, 978-1111580650
Students also viewed these Accounting questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



