Question
1.10 (a) For the reaction: CO + }O, C, What is the enthalpy of reaction (AH) at 298 K? (b) A fuel gas, with
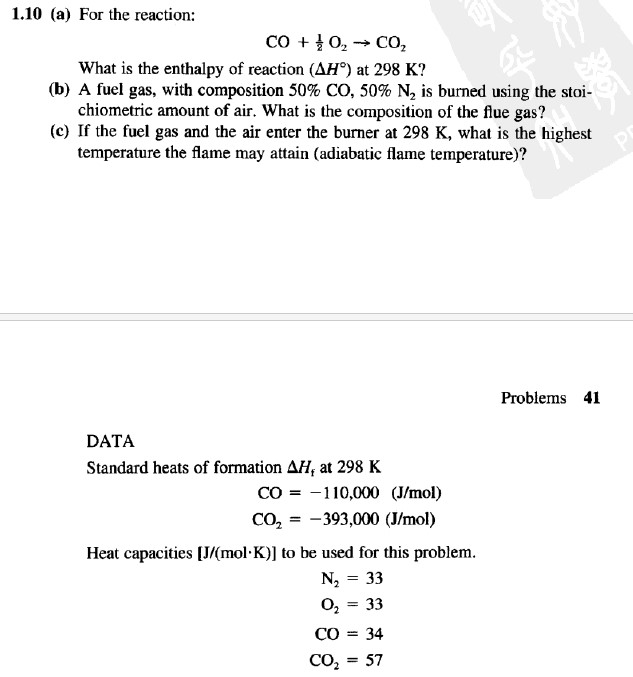
1.10 (a) For the reaction: CO + }O, C, What is the enthalpy of reaction (AH) at 298 K? (b) A fuel gas, with composition 50% CO, 50% N2 is burned using the stoi- chiometric amount of air. What is the composition of the flue gas? (c) If the fuel gas and the air enter the burner at 298 K, what is the highest temperature the flame may attain (adiabatic flame temperature)? DATA Standard heats of formation AH, at 298 K CO = 110,000 (J/mol) CO = -393,000 (J/mol) Heat capacities [J/(molK)] to be used for this problem. N = 33 O = 33 CO = 34 CO = 57 Problems 41
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Combustion of CO and Air Enthalpy Flue Gas and Adiabatic Flame Temperature This problem explores the combustion of carbon monoxide CO with air and cal...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Thermodynamics An Engineering Approach
Authors: Yunus A. Cengel, Michael A. Boles
8th edition
73398179, 978-0073398174
Students also viewed these Mechanical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



