Question
11.11 A junior engineer is assigned to study the distillation column that is used for the separation of isopropanol (1) and n -hexane (2).
\ 11.11 A junior engineer is assigned to study the distillation column that is used for\ the separation of isopropanol (1) and
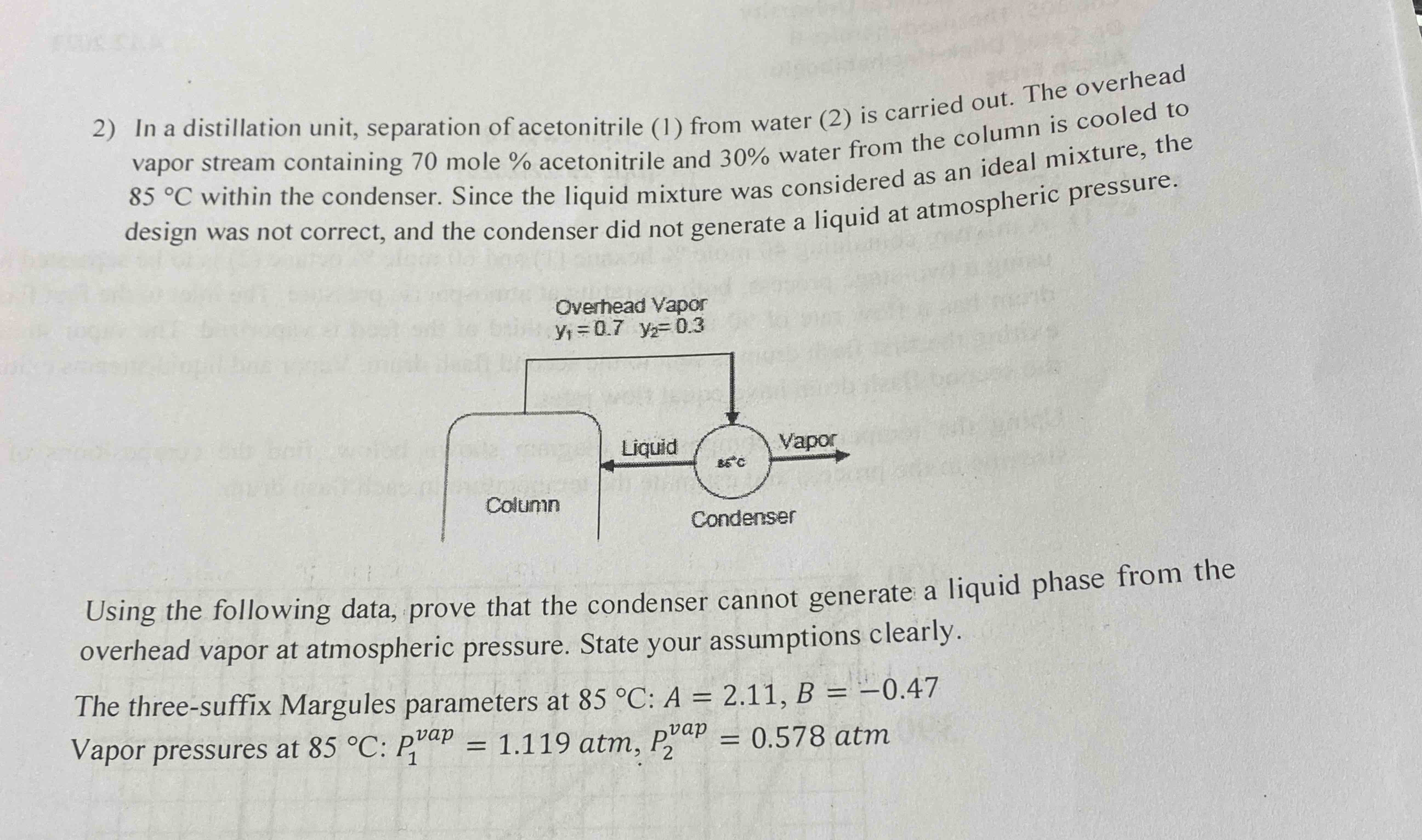
n-hexane (2). On his/her first day in theIn a distillation unit, separation of acetonitrile (1) from water (2) is carried out. The overhead\ vapor stream containing 70 mole
%acetonitrile and
30%water from the column is cooled to\
85\\\\deg Cwithin the condenser. Since the liquid mixture was considered as an ideal mixture, the\ design was not correct, and the condenser did not generate a liquid at atmospheric pressure.\ Using the following data, prove that the condenser cannot generate a liquid phase from the\ overhead vapor at atmospheric pressure. State your assumptions clearly.\ The three-suffix Margules parameters at
85\\\\deg C:A=2.11,B=-0.47\ Vapor pressures at
85\\\\deg C:P_(1)^(vap )=1.119atm,P_(2)^(vap )=0.578atm\ job, after going over his/her calculations for the reboiler, the engineer rushes to\ his/her boss' office out of breath and says,\ 'There's something wrong! We must change the operating conditions of\ the reboiler quickly. I double-checked my calculations and there shouldn't\ be any vapor produced under these conditions!"\ "What are the existing operating conditions?" the boss asks.\ The engineer replies, "the stream entering the reboiler contains
15mol%\ isopropanol, and the operating temperature and pressure are
335Kand\ 0.944 bar, respectively."\ Realizing that the engineer has used Raoult's law in his/her calculations, the\ boss laughs and pats him/her on the shoulder. "Don't worry" the boss says,\ "everything is alright. But it seems to me that you were daydreaming in Dr.\ Tosun's thermodynamics classes!"\ Show that the use of Raoult's law does indeed lead to no vapor generation.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


