Answered step by step
Verified Expert Solution
Question
1 Approved Answer
11,13,15 and 17 11. According to Section 2.6, acid leaching is not suitable for ores containing high levels of CaCO3 or MgCO3 because these carbonates
11,13,15 and 17 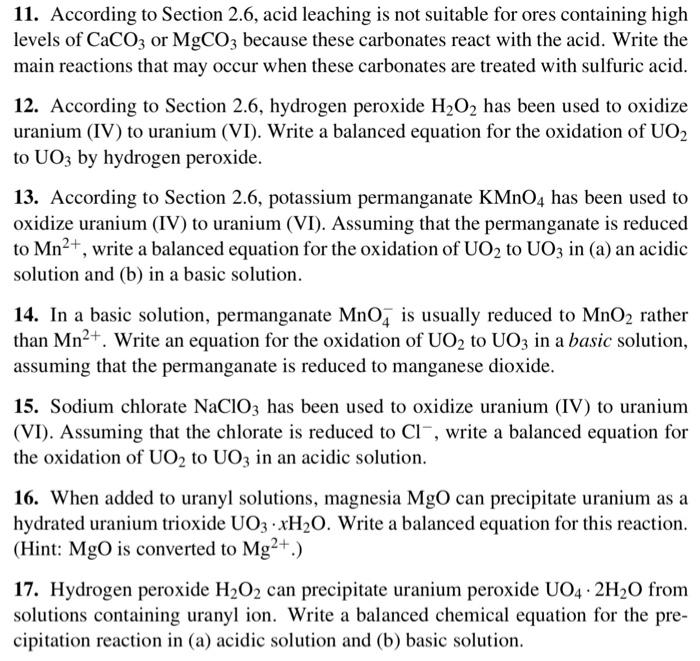
11. According to Section 2.6, acid leaching is not suitable for ores containing high levels of CaCO3 or MgCO3 because these carbonates react with the acid. Write the main reactions that may occur when these carbonates are treated with sulfuric acid. 12. According to Section 2.6, hydrogen peroxide H2O2 has been used to oxidize uranium (IV) to uranium (VI). Write a balanced equation for the oxidation of UO2 to UO3 by hydrogen peroxide. 13. According to Section 2.6, potassium permanganate KMnO4 has been used to oxidize uranium (IV) to uranium (VI). Assuming that the permanganate is reduced to Mn2+, write a balanced equation for the oxidation of UO2 to UO3 in (a) an acidic solution and (b) in a basic solution. 14. In a basic solution, permanganate MnO4is usually reduced to MnO2 rather than Mn2+. Write an equation for the oxidation of UO2 to UO3 in a basic solution, assuming that the permanganate is reduced to manganese dioxide. 15. Sodium chlorate NaClO3 has been used to oxidize uranium (IV) to uranium (VI). Assuming that the chlorate is reduced to Cl, write a balanced equation for the oxidation of UO2 to UO3 in an acidic solution. 16. When added to uranyl solutions, magnesia MgO can precipitate uranium as a hydrated uranium trioxide UO3xH2O. Write a balanced equation for this reaction. (Hint: MgO is converted to Mg2+.) 17. Hydrogen peroxide H2O2 can precipitate uranium peroxide UO42H2O from solutions containing uranyl ion. Write a balanced chemical equation for the precipitation reaction in (a) acidic solution and (b) basic solution 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


