Answered step by step
Verified Expert Solution
Question
1 Approved Answer
11.7-1. Flash Vaporization of Multicomponent Feed. For the feed to the distillation tower of Example 11.1-1, calculate the following. (a) Dew point of feed and
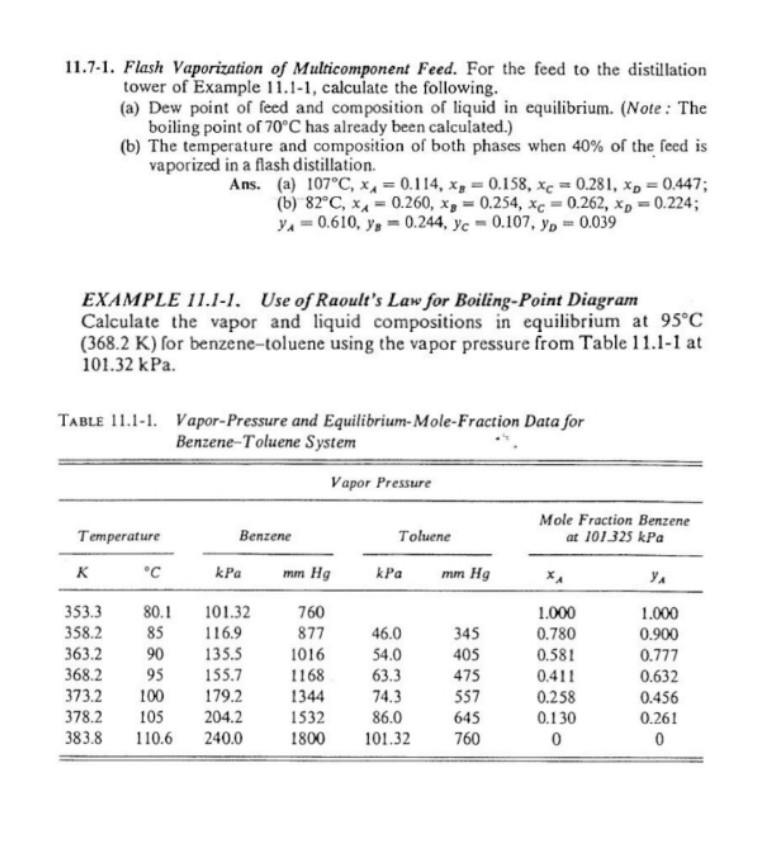
11.7-1. Flash Vaporization of Multicomponent Feed. For the feed to the distillation tower of Example 11.1-1, calculate the following. (a) Dew point of feed and composition of liquid in equilibrium. (Note: The boiling point of 70C has already been calculated.) (b) The temperature and composition of both phases when 40% of the feed is vaporized in a flash distillation. Ans. (a) 107C,xA=0.114,xB=0.158,xC=0.281,xD=0.447; (b) 82C,xA=0.260,xB=0.254,xC=0.262,xD=0.224; yA=0.610,yB=0.244,yC=0.107,yD=0.039 EXAMPLE 11.I-I. Use of Raoult's Law for Boiling-Point Diagram Calculate the vapor and liquid compositions in equilibrium at 95C ( 368.2K) for benzene-toluene using the vapor pressure from Table 11.1-1 at 101.32kPa
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


