Answered step by step
Verified Expert Solution
Question
1 Approved Answer
12 answer all parts of the question please According to the following reaction, how many grams of ammonium chloride are necessary to form 0.438 moles
12 

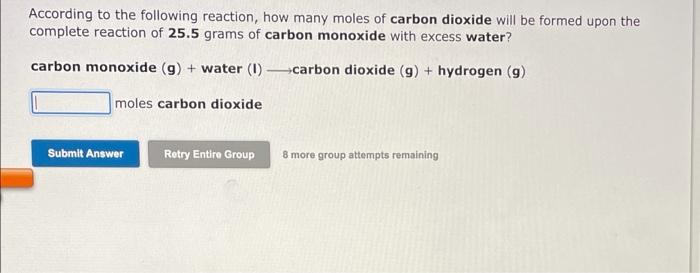
According to the following reaction, how many grams of ammonium chloride are necessary to form 0.438 moles hydrochloric acid? ammonium chloride (aq)-ammonia (9) + hydrochloric acid (aq) grams ammonium chloride led Submit Answer Retry Entire Group 8 more group attempts remaining According to the following reaction, how many grams of chlorine gas are necessary to form 0.914 moles phosphorus trichloride? phosphorus (P4) (s) + chlorine (g) phosphorus trichloride (1) grams chlorine gas Submit Answer Retry Entire Group B more group attempts remaining According to the following reaction, how many moles of carbon dioxide will be formed upon the complete reaction of 25.5 grams of carbon monoxide with excess water? carbon monoxide (9) + water (1) carbon dioxide (9) + hydrogen (9) moles carbon dioxide Submit Answer Retry Entire Group 8 more group attempts remaining answer all parts of the question please



Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


