Answered step by step
Verified Expert Solution
Question
1 Approved Answer
12. Show that the van der Waals equation leads to values of Z < 1 and Z> 1, and identify the conditions for which
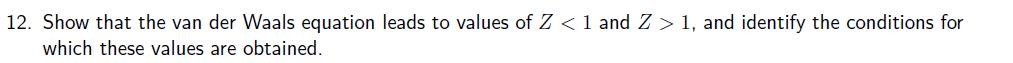

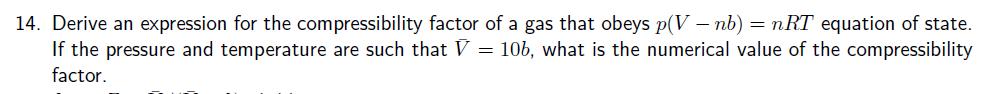
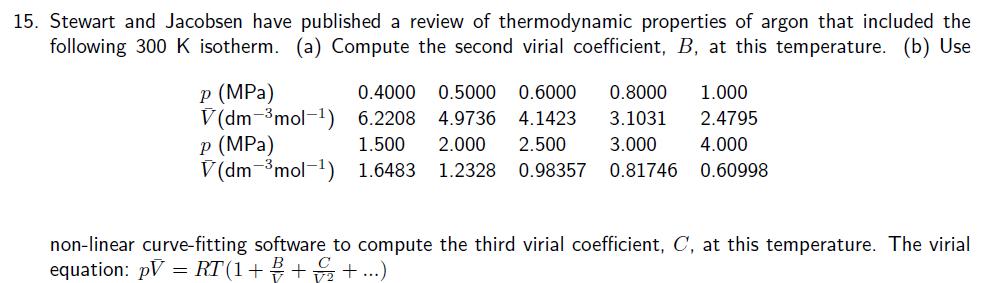
12. Show that the van der Waals equation leads to values of Z < 1 and Z> 1, and identify the conditions for which these values are obtained. 13. The following equations of state are occasionally used for approximate calculations on gases: (gas A) pV=RT (1+b/V), (gas B) p(V - b) = RT. Assuming that there were gases that actually obeyed theses equations of state, would it be possible to liquefy either gas A or B? Would they have a critical temperature? Explain your answer. 14. Derive an expression for the compressibility factor of a gas that obeys p(V - nb) = nRT equation of state. If the pressure and temperature are such that V = 10b, what is the numerical value of the compressibility factor. 15. Stewart and Jacobsen have published a review of thermodynamic properties of argon that included the following 300 K isotherm. (a) Compute the second virial coefficient, B, at this temperature. (b) Use P (MPa) V(dm-mol-1) p (MPa) 0.4000 0.5000 0.6000 6.2208 4.9736 4.1423 1.500 2.000 2.500 0.8000 1.000 3.1031 2.4795 3.000 4.000 V(dm mol 1) 1.6483 1.2328 0.98357 0.81746 0.60998 non-linear curve-fitting software to compute the third virial coefficient, C, at this temperature. The virial equation: pV = RT(1+++...)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


