Answered step by step
Verified Expert Solution
Question
1 Approved Answer
12. The treatment of galena with HNO3 produces a gas that is (A) paramagnetic (C) an acidic oxide (B) bent in geometry (D) colorless

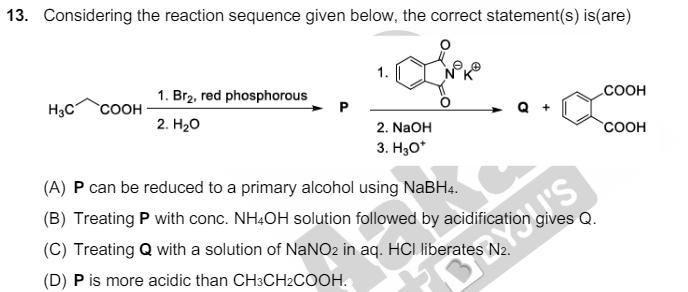
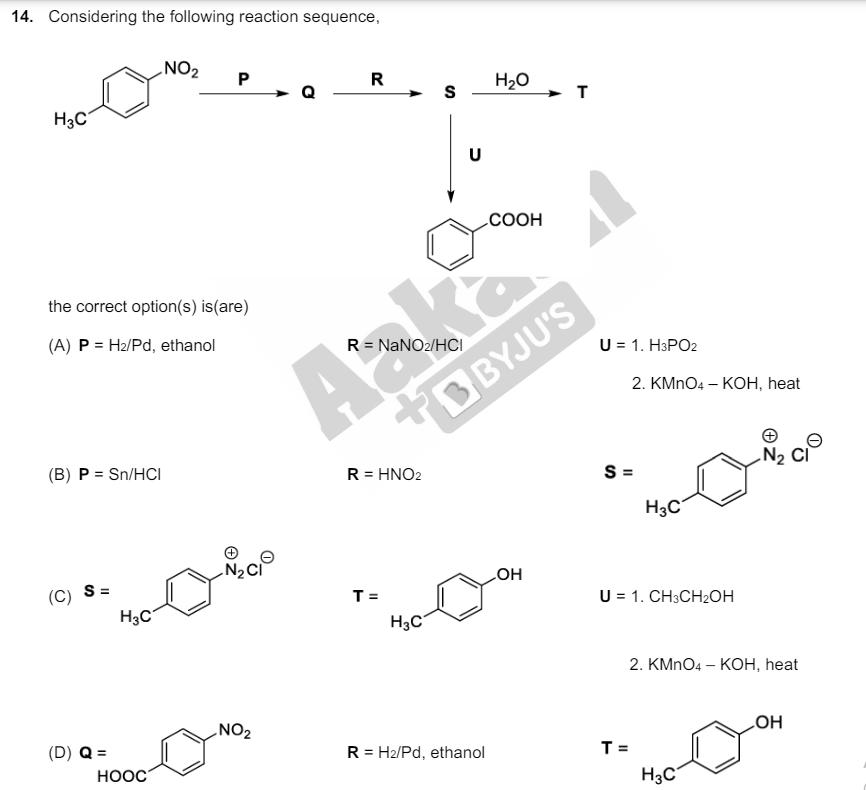
12. The treatment of galena with HNO3 produces a gas that is (A) paramagnetic (C) an acidic oxide (B) bent in geometry (D) colorless 13. Considering the reaction sequence given below, the correct statement(s) is (are) 1. Br2, red phosphorous H3C COOH 2. HO 2. NaOH 3. HO* (A) P can be reduced to a primary alcohol using NaBH4. (B) Treating P with conc. NH4OH solution followed by acidification gives Q. Cation (C) Treating Q with a solution of NaNO2 in aq. HCI liberates N2. (D) P is more acidic than CH3CH2COOH. COOH COOH 14. Considering the following reaction sequence, NO2 P R HO S T H3C U the correct option(s) is (are) (A) P H2/Pd, ethanol R = NaNO2/HCI (B) P = Sn/HCI Ak R=HNO2 COOH BBYJU'S (C) S = H3C N20 T = (D) Q = HOOC H3C U = 1. H3PO2 2. KMnO4 KOH, heat S = H3C OH U 1. CH3CH2OH 2. KMnO4 - KOH, heat NO2 = R H2/Pd, ethanol T = H3C
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


