1.26cd. Molecular diffusion through a gas-liquid interface Ammonia, NH3, is being selectively removed from an air-NH, mixture by absorption into water. In this steady-state
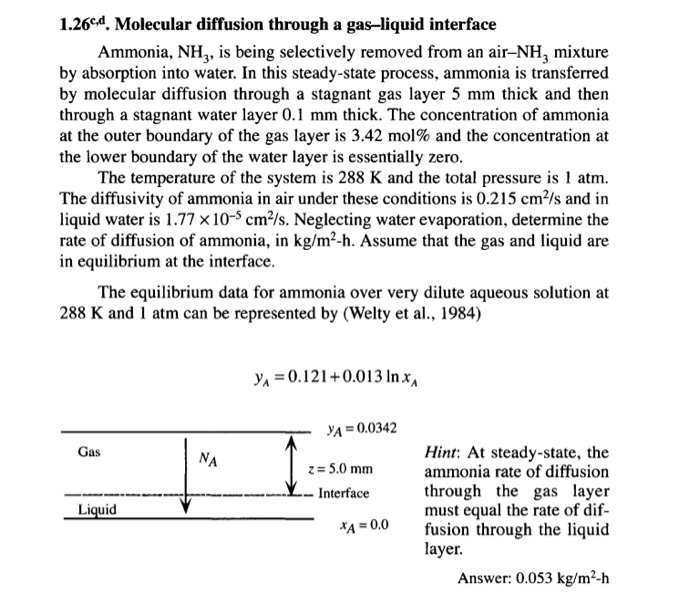
1.26cd. Molecular diffusion through a gas-liquid interface Ammonia, NH3, is being selectively removed from an air-NH, mixture by absorption into water. In this steady-state process, ammonia is transferred by molecular diffusion through a stagnant gas layer 5 mm thick and then through a stagnant water layer 0.1 mm thick. The concentration of ammonia at the outer boundary of the gas layer is 3.42 mol % and the concentration at the lower boundary of the water layer is essentially zero. The temperature of the system is 288 K and the total pressure is 1 atm. The diffusivity of ammonia in air under these conditions is 0.215 cm/s and in liquid water is 1.77 x 10-5 cm/s. Neglecting water evaporation, determine the rate of diffusion of ammonia, in kg/m-h. Assume that the gas and liquid are in equilibrium at the interface. The equilibrium data for ammonia over very dilute aqueous solution at 288 K and 1 atm can be represented by (Welty et al., 1984) Gas Liquid NA y = 0.121+0.013 ln x YA = 0.0342 z = 5.0 mm Interface *A=0.0 Hint: At steady-state, the ammonia rate of diffusion through the gas layer must equal the rate of dif- fusion through the liquid layer. Answer: 0.053 kg/m-h
Step by Step Solution
3.39 Rating (165 Votes )
There are 3 Steps involved in it
Step: 1

See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


