Answered step by step
Verified Expert Solution
Question
1 Approved Answer
13. 7 mol of a certain monoatomic ideal gas undergoes a temperature increase of 40K at constant pressure. The increase in the internal energy
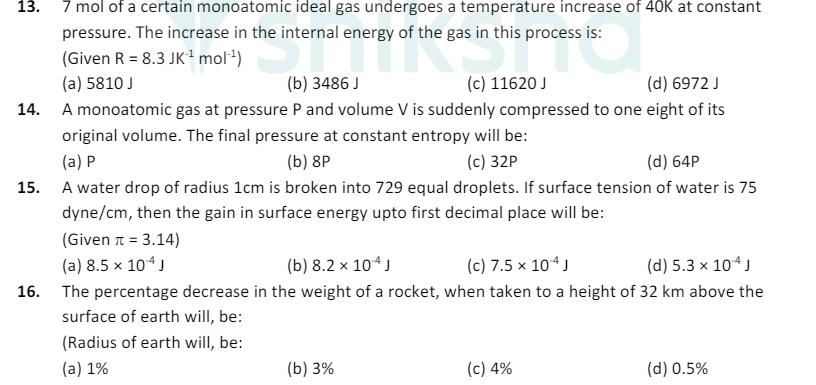
13. 7 mol of a certain monoatomic ideal gas undergoes a temperature increase of 40K at constant pressure. The increase in the internal energy of the gas in this process is: (Given R 8.3 JK mol) (a) 5810 J (b) 3486 J (c) 11620 J (d) 6972 J 14. A monoatomic gas at pressure P and volume V is suddenly compressed to one eight of its original volume. The final pressure at constant entropy will be: (a) P (b) 8P (c) 32P (d) 64P 15. A water drop of radius 1cm is broken into 729 equal droplets. If surface tension of water is 75 dyne/cm, then the gain in surface energy upto first decimal place will be: (Given = 3.14) (a) 8.5 x 104 J surface of earth will, be: (b) 8.2 104 J (c) 7.5 x 104 J (d) 5.3 x 104 J 16. The percentage decrease in the weight of a rocket, when taken to a height of 32 km above the (Radius of earth will, be: (a) 1% (b) 3% (c) 4% (d) 0.5%
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


