Answered step by step
Verified Expert Solution
Question
1 Approved Answer
13. A mixture of air and a hydrocarbon fuel whose average composition is indi- cated by CH19 undergoes complete combustion. Twice as much air
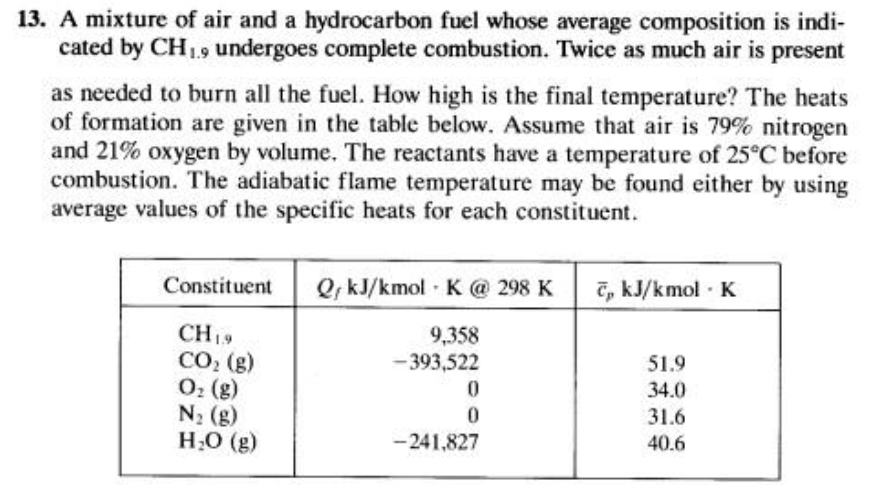
13. A mixture of air and a hydrocarbon fuel whose average composition is indi- cated by CH19 undergoes complete combustion. Twice as much air is present as needed to burn all the fuel. How high is the final temperature? The heats of formation are given in the table below. Assume that air is 79% nitrogen and 21% oxygen by volume. The reactants have a temperature of 25C before combustion. The adiabatic flame temperature may be found either by using average values of the specific heats for each constituent. Constituent CH19 CO (g) O (g) N (g) HO (g) QkJ/kmol K @ 298 K , kJ/kmol. K 9,358 -393,522 0 0 -241,827 51.9 34.0 31.6 40.6
Step by Step Solution
★★★★★
3.47 Rating (154 Votes )
There are 3 Steps involved in it
Step: 1
To solve this problem we can use the adiabatic flame temperature formula Tfinal Qcombustion Cp M Tin...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


