Answered step by step
Verified Expert Solution
Question
1 Approved Answer
13 b 13. Consider the following reaction: CH(g) + HBr (g) CH3Br (g) (CH3Br has 2 hydrogens attached to one carbon, and one hydrogen and
13 b

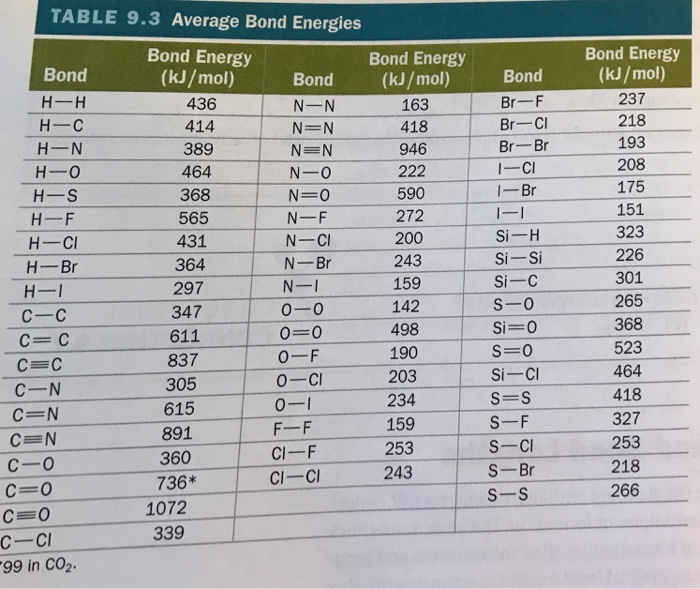
13. Consider the following reaction: CH(g) + HBr (g) CH3Br (g) (CH3Br has 2 hydrogens attached to one carbon, and one hydrogen and a bromine attached to the other carbon (and the two carbons are attached to each other)) (a) Draw the Lewis structures for HBr and CH,Br in the reaction above: H-Bri H-C=C-H H (b) What two electrons are lost to form Ni+? H -C=CIH 11 Br (b) Calculate the enthalpy of the overall reaction (AH) using the bond dissociation energies in your book, and using the structure of CH below (Use 285 kJ/mol as the bond dissociation energy for the C-Br bond). 7/148 14. (a) Draw the complete energy orbital diagram for Ni (with correct placement of relative energy levels of the orbitals); fill in the orbitals with the Ni electrons.
Step by Step Solution
★★★★★
3.48 Rating (158 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


