Question
13. Which of the following would be the most likely to undergo an E1 elimination reaction with a very strong base, such as the (CH3)3CO
13. Which of the following would be the most likely to undergo an E1 elimination reaction with a very strong base, such as the (CH3)3CO ion?

24. Identify the compound in each of the following pairs that react at the faster rate in an SN1
reaction:
(a) Isopropyl bromide or isobutyl bromide
(b) Cyclopentyl iodide or 1-methylcyclopentyl iodide
(c) Cyclopentyl bromide or 1-bromo-2,2-dimethylpropane
(d) tert-Butyl chloride or tert-butyl iodide
26. Predict whether the following reactions are more likely to undergo elimination or substitution.
Identify the mechanism of the dominant reaction (E1 versus E2; SN1 versus SN2).
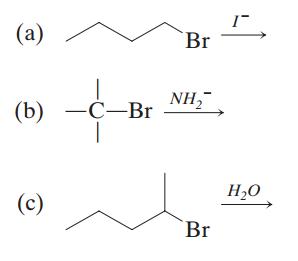
Br Br Br B C
Step by Step Solution
3.44 Rating (157 Votes )
There are 3 Steps involved in it
Step: 1
13 3 alkyl halide undergoes E1 eliminatio...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Auditing A Practical Approach with Data Analytics
Authors: Raymond N. Johnson, Laura Davis Wiley, Robyn Moroney, Fiona Campbell, Jane Hamilton
1st edition
1119401747, 978-1119401742
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



