Answered step by step
Verified Expert Solution
Question
1 Approved Answer
13.5. As pointed out in the Process Description, the water-gas shift reaction (Equation 13.2) occurs in the reformer along with the reforming reaction (Equation
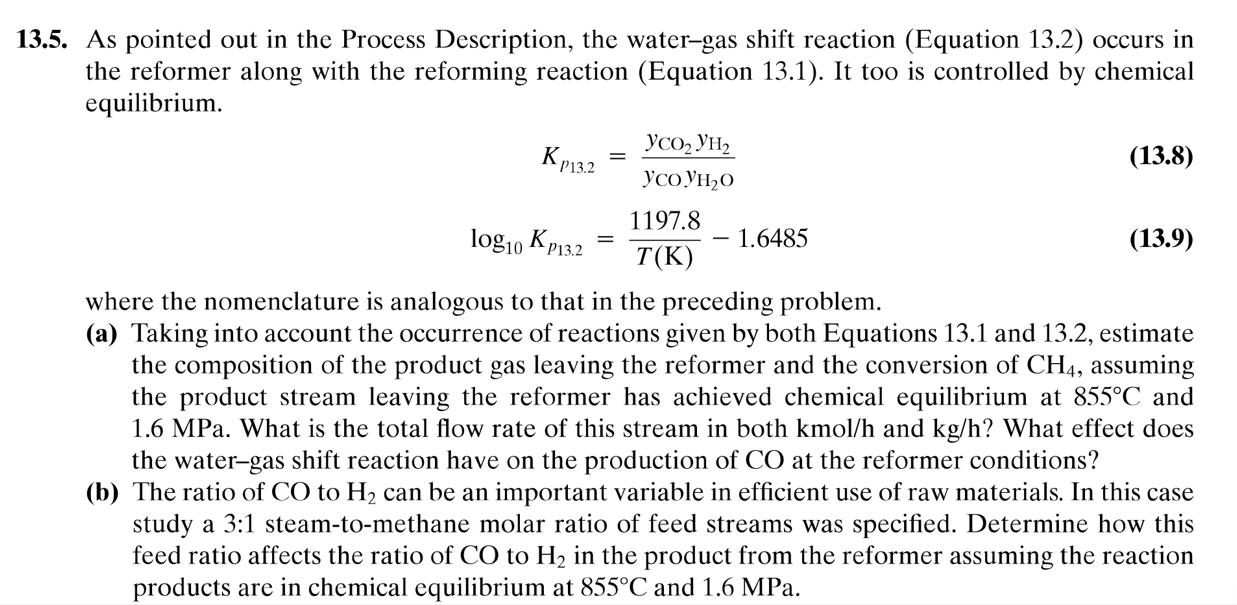
13.5. As pointed out in the Process Description, the water-gas shift reaction (Equation 13.2) occurs in the reformer along with the reforming reaction (Equation 13.1). It too is controlled by chemical equilibrium. KP13.2 = log10 KP13.2 2 20 1197.8 T(K) - 1.6485 (13.8) (13.9) where the nomenclature is analogous to that in the preceding problem. (a) Taking into account the occurrence of reactions given by both Equations 13.1 and 13.2, estimate the composition of the product gas leaving the reformer and the conversion of CH4, assuming the product stream leaving the reformer has achieved chemical equilibrium at 855C and 1.6 MPa. What is the total flow rate of this stream in both kmol/h and kg/h? What effect does the water-gas shift reaction have on the production of CO at the reformer conditions? (b) The ratio of CO to H can be an important variable in efficient use of raw materials. In this case study a 3:1 steam-to-methane molar ratio of feed streams was specified. Determine how this feed ratio affects the ratio of CO to H in the product from the reformer assuming the reaction products are in chemical equilibrium at 855C and 1.6 MPa.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


