Question
1.38 g of NaCl (molar mass 58.44 g/mol) as dissolved in 43.3 mL of water. Assuming molar conductivity A ion activity coefficient, a (mS/mol/cm)
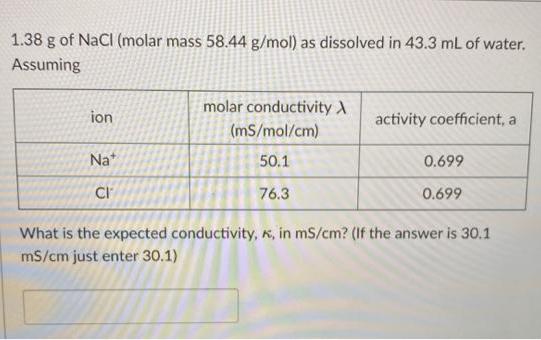
1.38 g of NaCl (molar mass 58.44 g/mol) as dissolved in 43.3 mL of water. Assuming molar conductivity A ion activity coefficient, a (mS/mol/cm) Na* 50.1 0.699 CI 76.3 0.699 What is the expected conductivity, K, in mS/cm? (If the answer is 30.1 mS/cm just enter 30.1)
Step by Step Solution
3.41 Rating (157 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Statistics And Data Analysis For Financial Engineering
Authors: David Ruppert
1st Edition
1461427495, 978-1461427490
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



