Question
1-4. A sample of water having a pH of 7.2 has the following concentrations of ions: Ca2+ 40 mg/L Mg+ 10 mg/L Na 11.8
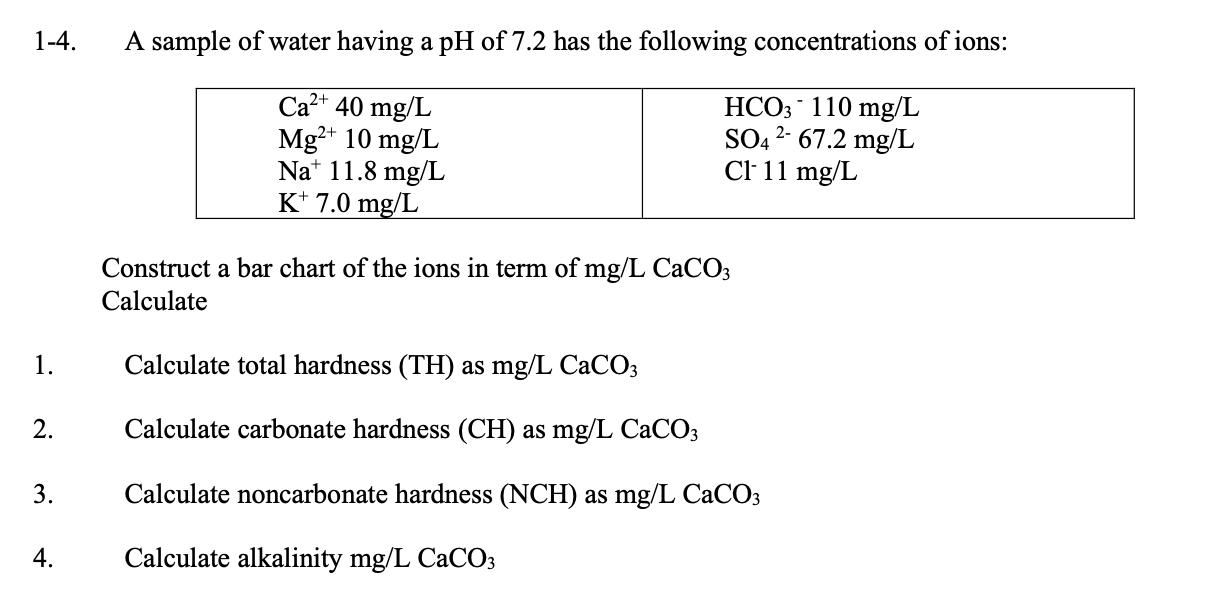
1-4. A sample of water having a pH of 7.2 has the following concentrations of ions: Ca2+ 40 mg/L Mg+ 10 mg/L Na 11.8 mg/L K+ 7.0 mg/L Construct a bar chart of the ions in term of mg/L CaCO3 Calculate HCO3 110 mg/L SO4267.2 mg/L Cl- 11 mg/L 1. Calculate total hardness (TH) as mg/L CaCO3 2. Calculate carbonate hardness (CH) as mg/L CaCO3 3. Calculate noncarbonate hardness (NCH) as mg/L CaCO3 4. Calculate alkalinity mg/L CaCO3
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Applied Regression Analysis And Other Multivariable Methods
Authors: David G. Kleinbaum, Lawrence L. Kupper, Azhar Nizam, Eli S. Rosenberg
5th Edition
1285051084, 978-1285963754, 128596375X, 978-1285051086
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



