Answered step by step
Verified Expert Solution
Question
1 Approved Answer
14) Answer the following questions using the phase diagram of the iron-carbon system. a) A plain carbon steel contains 70 wt % ferrite and
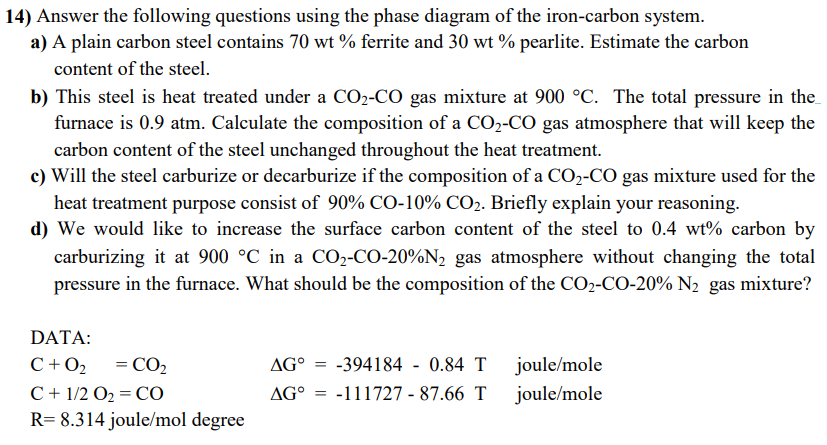
14) Answer the following questions using the phase diagram of the iron-carbon system. a) A plain carbon steel contains 70 wt % ferrite and 30 wt % pearlite. Estimate the carbon content of the steel. b) This steel is heat treated under a CO2-CO gas mixture at 900 C. The total pressure in the furnace is 0.9 atm. Calculate the composition of a CO2-CO gas atmosphere that will keep the carbon content of the steel unchanged throughout the heat treatment. c) Will the steel carburize or decarburize if the composition of a CO2-CO gas mixture used for the heat treatment purpose consist of 90% CO-10% CO2. Briefly explain your reasoning. d) We would like to increase the surface carbon content of the steel to 0.4 wt% carbon by carburizing it at 900 C in a CO2-CO-20%N2 gas atmosphere without changing the total pressure in the furnace. What should be the composition of the CO2-CO-20% N2 gas mixture? DATA: C+02 = CO C+1/2 O2 CO AG = AG = -394184 0.84 T -111727 87.66 T - joule/mole joule/mole R=8.314 joule/mol degree
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


