Question
Consider an amino acid with an extra amino group in the side chain: arginine, add NaOH to achieve its dissociation, to dissociate first. H,N-CH-C-OH
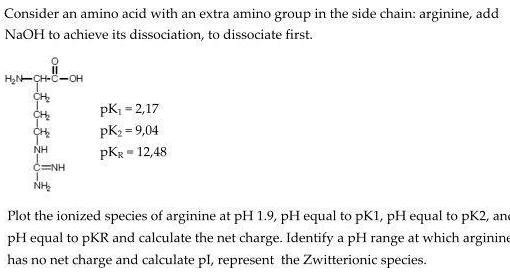
Consider an amino acid with an extra amino group in the side chain: arginine, add NaOH to achieve its dissociation, to dissociate first. H,N-CH-C-OH CH pK1 = 2,17 pK2 = 9,04 pKg = 12,48 NH C=NH NH Plot the ionized species of arginine at pH 1.9, pH equal to pK1, pH equal to pK2, and e pH equal to pKR and calculate the net charge. Identify a pH range at which arginine has no net charge and calculate pl, represent the Zwitterionic species.
Step by Step Solution
3.29 Rating (140 Votes )
There are 3 Steps involved in it
Step: 1
Therefore There is the dissociation of arginine Lets suppose that we have a mixture of A and B A and ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Biochemistry Concepts and Connections
Authors: Dean R. Appling, Spencer J. Anthony Cahill, Christopher K. Mathews
1st edition
321839927, 978-0133853490, 133853497, 978-0321839923
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



