Question
15. The half-life of carbon-14 is 5760 years. Assuming you start with 100 percent of carbon-14, what is the expression for the percent, P(t),
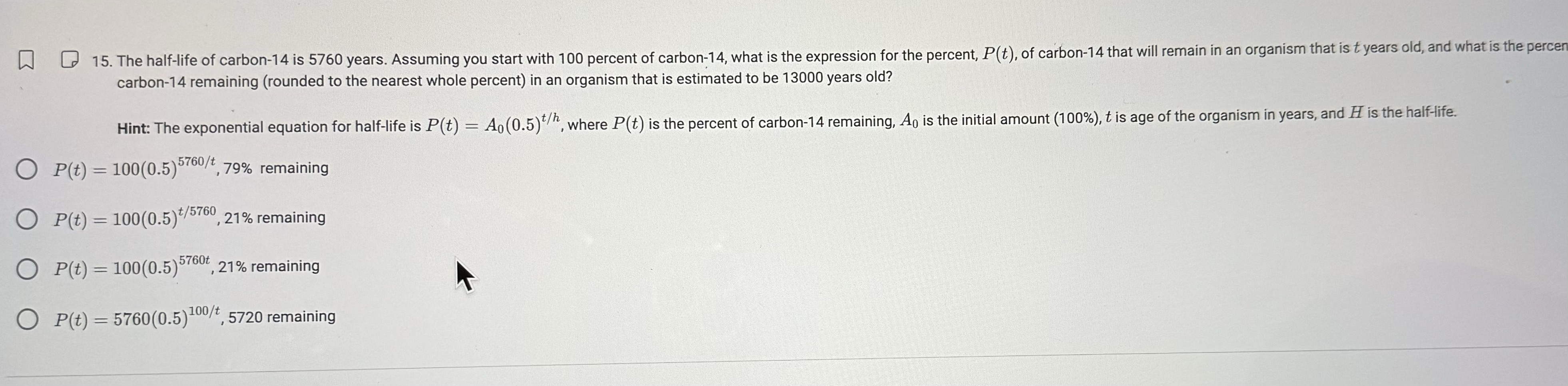
15. The half-life of carbon-14 is 5760 years. Assuming you start with 100 percent of carbon-14, what is the expression for the percent, P(t), of carbon-14 that will remain in an organism that is t years old, and what is the percen carbon-14 remaining (rounded to the nearest whole percent) in an organism that is estimated to be 13000 years old? Hint: The exponential equation for half-life is P(t) = A0 (0.5)t/h, where P(t) is the percent of carbon-14 remaining, A is the initial amount (100%), t is age of the organism in years, and H is the half-life. OP(t)=100(0.5)5 P(t) = 100(0.5)5760/t 79% remaining 100(0.5)*/5760,21% remaining OP(t)=100(0.5)* OP(t)=100(0.5) 5760, 21% remaining OP(t)=5760(0.5) 100/+, 5720 remaining
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Business Statistics A Decision Making Approach
Authors: David F. Groebner, Patrick W. Shannon, Phillip C. Fry
9th Edition
013302184X, 978-0133021844
Students also viewed these Mathematics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App