Question
16. Strontium-90 undergoes beta decay and has a half-life of 28 years. a) Write the nuclear equation for the beta decay of strontium-90. (2)
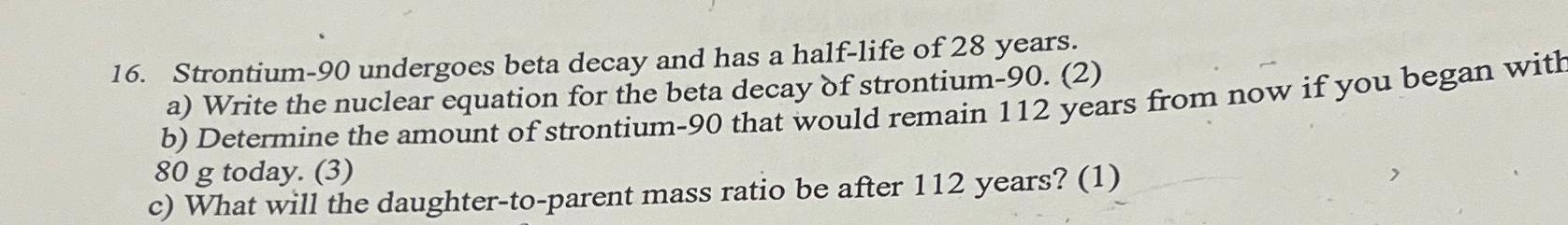
16. Strontium-90 undergoes beta decay and has a half-life of 28 years. a) Write the nuclear equation for the beta decay of strontium-90. (2) b) Determine the amount of strontium-90 that would remain 112 years from now if you began with 80 g today. (3) c) What will the daughter-to-parent mass ratio be after 112 years? (1)
Step by Step Solution
There are 3 Steps involved in it
Step: 1
a The nuclear equation for the beta decay of strontium90 is 9038Sr 9039Y e antie b To determin...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Introductory Chemistry version 1.0
Authors: David W. Ball
1st edition
1453327657, 978-1453327654
Students also viewed these Mathematics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



