Question
17. For each set of common laboratory solvents, identify the compound that is more acidic. H,C-OH H,C CH methanol acetone vs. I H-c-CI dichloromethane
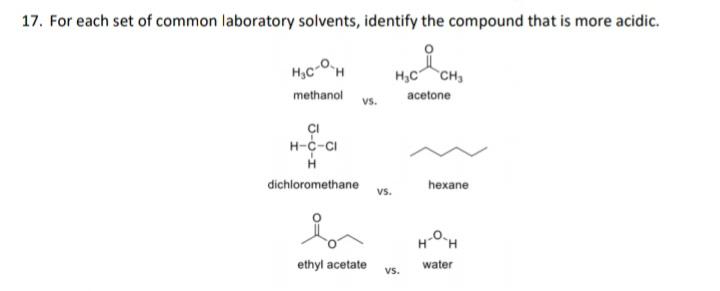
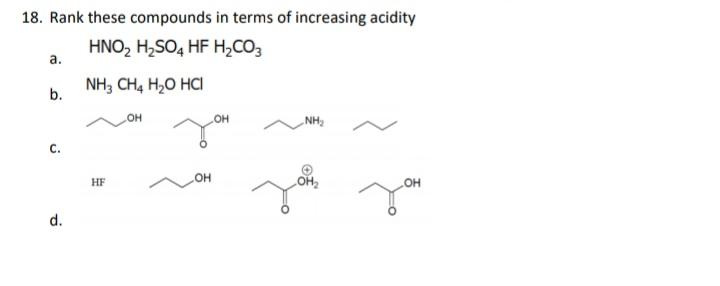
17. For each set of common laboratory solvents, identify the compound that is more acidic. H,C-OH H,C CH methanol acetone vs. I H-c-CI dichloromethane hexane vs. H-OH ethyl acetate water vs. 18. Rank these compounds in terms of increasing acidity HNO, H,SO, HF H,CO3 . NH, CH, H,O HCI b. HO NH, . HO HF OH d.
Step by Step Solution
3.35 Rating (161 Votes )
There are 3 Steps involved in it
Step: 1
17 A CH3OH since it has a more polar OH b...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Organic Chemistry
Authors: Graham Solomons, Craig Fryhle, Scott Snyder
11th edition
1118133579, 978-1118133576
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



