Answered step by step
Verified Expert Solution
Question
1 Approved Answer
17. What is the mass percent (%) for O in SO2? (b) 45.41 (a) 38.09 (c) 50.00 (d) 53.86 (e) 56.43 18. Which of
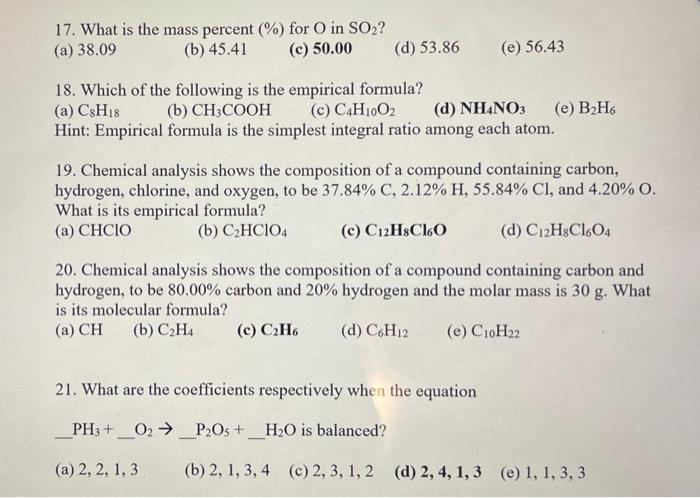
17. What is the mass percent (%) for O in SO2? (b) 45.41 (a) 38.09 (c) 50.00 (d) 53.86 (e) 56.43 18. Which of the following is the empirical formula? (a) CSH18 (c) C4H1002 Hint: Empirical formula is the simplest integral ratio among each atom. (b) CH3COOH (d) NH4NO3 () -6 19. Chemical analysis shows the composition of a compound containing carbon, hydrogen, chlorine, and oxygen, to be 37.84% C, 2.12% H, 55.84% Cl, and 4.20% O. What is its empirical formula? () HCIO (b) C2HCIO4 (c) C12HsCl6O (d) C12H&Cl6O4 20. Chemical analysis shows the composition of a compound containing carbon and hydrogen, to be 80.00% carbon and 20% hydrogen and the molar mass is 30 g. What is its molecular formula? (a) CH (b) C2H4 (c) C2H6 (d) C6H12 (e) C10H22 21. What are the coefficients respectively when the equation PH3 + O2 P2Os +_H2O is balanced? - (a) 2, 2, 1, 3 (b) 2, 1, 3, 4 (c) 2, 3, 1, 2 (d) 2, 4, 1, 3 (e) 1, 1, 3, 3
Step by Step Solution
★★★★★
3.52 Rating (155 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Document Format ( 2 attachments)
63616494ae974_235700.pdf
180 KBs PDF File
63616494ae974_235700.docx
120 KBs Word File
Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


