Question
18. Given the following data for boron trichloride at 25 C, calculate S for BCls(D. AHP BCl5() =-427.2 kJ/mol; AHP BCh(g) =-403.8 kJ/mol; S
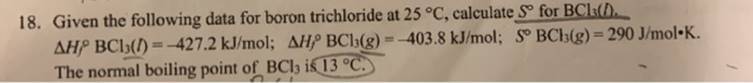
18. Given the following data for boron trichloride at 25 C, calculate S for BCls(D. AHP BCl5() =-427.2 kJ/mol; AHP BCh(g) =-403.8 kJ/mol; S BCb(g)= 290 J/mol-K. The normal boiling point of BCl ik 13 C. %3D !3!
Step by Step Solution
3.46 Rating (156 Votes )
There are 3 Steps involved in it
Step: 1
Gimen A ket bas A 4272 KJmol a Jmolk 4038 KJ mo 290 J lamolk No...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
OM operations management
Authors: David Alan Collier, James R. Evans
5th edition
1285451376, 978-1285451374
Students also viewed these Accounting questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



