Answered step by step
Verified Expert Solution
Question
1 Approved Answer
19. Rank in terms of decreasing acidity OH 20. What is the most acidic group of protons on methyl acetate? (This is a flammable
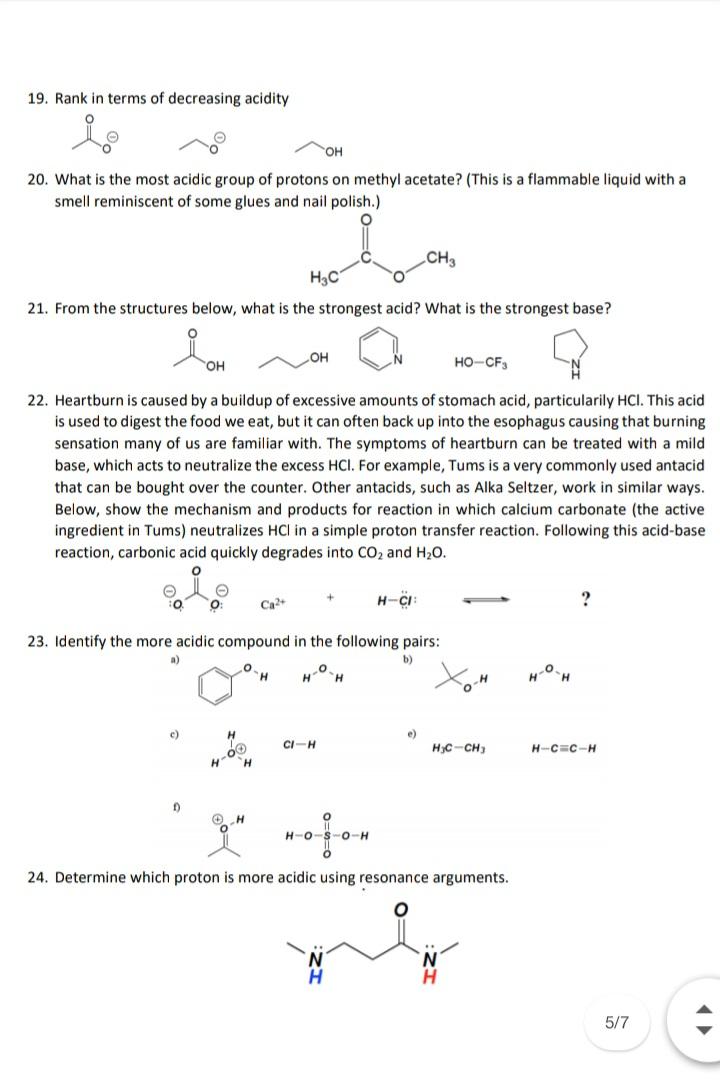
19. Rank in terms of decreasing acidity OH 20. What is the most acidic group of protons on methyl acetate? (This is a flammable liquid with a smell reminiscent of some glues and nail polish.) CH3 H3C 21. From the structures below, what is the strongest acid? What is the strongest base? HO. HO-CF3 22. Heartburn is caused by a buildup of excessive amounts of stomach acid, particularily HCI. This acid is used to digest the food we eat, but it can often back up into the esophagus causing that burning sensation many of us are familiar with. The symptoms of heartburn can be treated with a mild base, which acts to neutralize the excess HCI. For example, Tums is a very commonly used antacid that can be bought over the counter. Other antacids, such as Alka Seltzer, work in similar ways. Below, show the mechanism and products for reaction in which calcium carbonate (the active ingredient in Tums) neutralizes HCI in a simple proton transfer reaction. Following this acid-base reaction, carbonic acid quickly degrades into CO2 and H20. Ca2+ H-CI 23. Identify the more acidic compound in the following pairs: a) b) H. H. c) e) CI-H H3C-CH) H-C=C-H H. H-0-S-O-H 24. Determine which proton is more acidic using resonance arguments. 5/7
Step by Step Solution
★★★★★
3.35 Rating (158 Votes )
There are 3 Steps involved in it
Step: 1
19 As we know that the stronger the acid the weaker is its c...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


