Answered step by step
Verified Expert Solution
Question
1 Approved Answer
199 TABLE 5.2 Data and Results for Specific Heat of Metal 1 Metal (Hot Water) Initial Temperature, T E 64.931 70 70 Trial 1
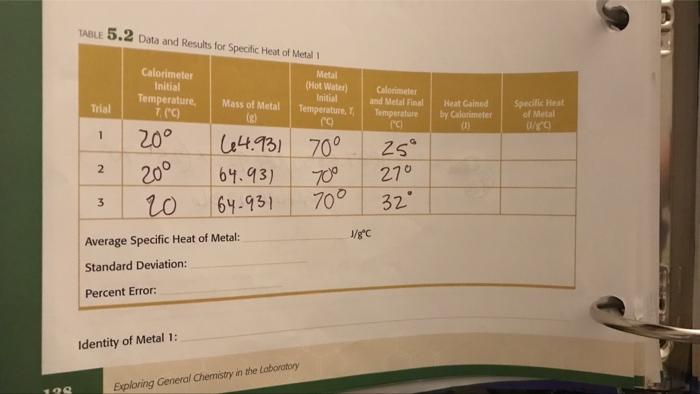
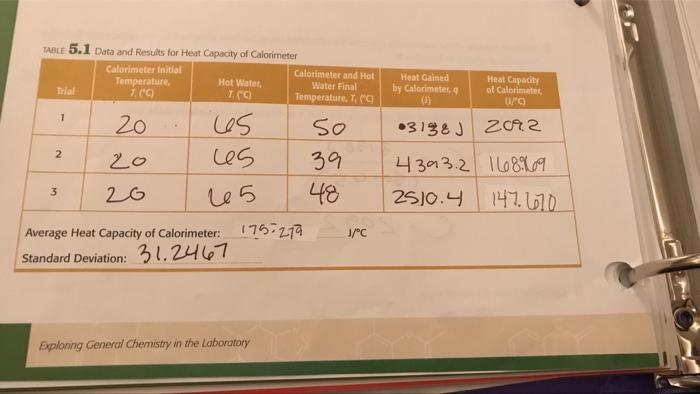
199 TABLE 5.2 Data and Results for Specific Heat of Metal 1 Metal (Hot Water) Initial Temperature, T E 64.931 70 70 Trial 1 2 3 Calorimeter Initial Temperature, 7. (C) 20 20 20 Mass of Metal (2) Identity of Metal 1: 64.93) 64-931 Average Specific Heat of Metal: Standard Deviation: Percent Error: Exploring General Chemistry in the Laboratory Calorimeter and Metal Final Temperature (c) J/gC 25 270 32 Heat Gained by Calorimeter B Specific Heat of Metal 0/80 TABLE 5.1 Data and Results for Heat Capacity of Calorimeter Calorimeter Initial Temperature, 7. (C) 20 Trial 1 2 3 20 20 Hot Water, TCC) los 65 25 Average Heat Capacity of Calorimeter: Standard Deviation: 31.2467 Calorimeter and Hot Water Final Temperature. T, ("C 50 39 48 175-279 Exploring General Chemistry in the Laboratory J/C Heat Gained by Calorimeter, q (1) 03138 J Heat Capacity of Calorimeter, 0/0) 2092 4393.2 168969 2510.4 147.670
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


