Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1.a 1.b For the following reaction, 12.9 grams of silicon tetrafluoride are allowed to react with 3.20grams of water . silicon tetrafluoride (s)+ water (I)
1.a

1.b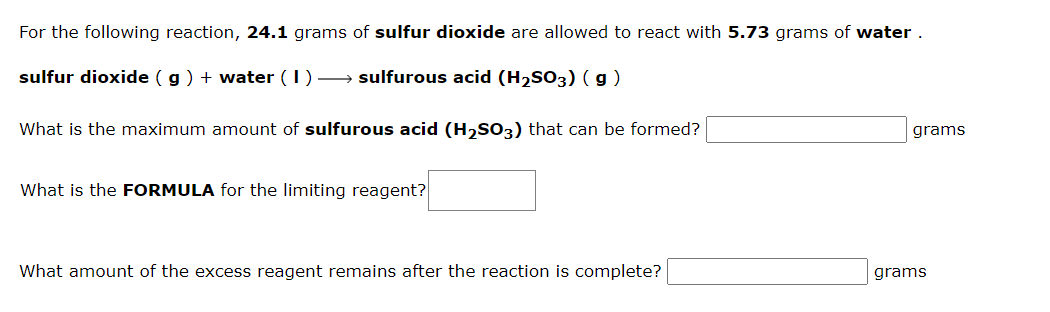
For the following reaction, 12.9 grams of silicon tetrafluoride are allowed to react with 3.20grams of water . silicon tetrafluoride (s)+ water (I) hydrofluoric acid ( aq ) + silicon dioxide ( s ) What is the maximum amount of hydrofluoric acid that can be formed? grams What is the FORMULA for the limiting reagent? What amount of the excess reagent remains after the reaction is complete? grams For the following reaction, 24.1 grams of sulfur dioxide are allowed to react with 5.73 grams of water . sulfurdioxide(g)+water(I)sulfurousacid(H2SO3)(g) What is the maximum amount of sulfurous acid (H2SO3) that can be formed? grams What is the FORMULA for the limiting reagent? What amount of the excess reagent remains after the reaction is complete? grams
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


