Question
2. Data for the gas-phase dissociation of sulfuryl chloride, SO,Cl, into chlorine, Cl, and sulfur dioxide, so, at 279.2 C are presented below. The
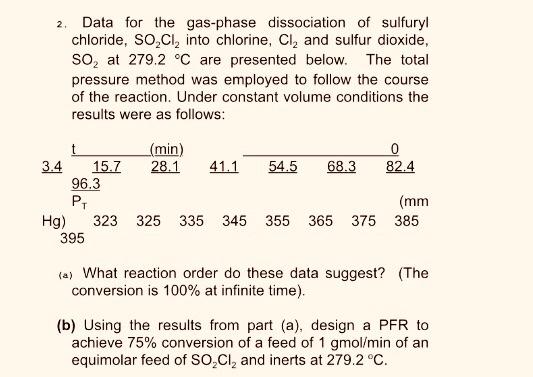
2. Data for the gas-phase dissociation of sulfuryl chloride, SO,Cl, into chlorine, Cl, and sulfur dioxide, so, at 279.2 C are presented below. The total pressure method was employed to follow the course of the reaction. Under constant volume conditions the results were as follows: (min) 28.1 41.1 54.5 3.4 15.7 96.3 68.3 82.4 (mm 345 Hg) 395 323 325 335 355 365 375 385 (a) What reaction order do these data suggest? (The conversion is 100% at infinite time). (b) Using the results from part (a), design a PFR to achieve 75% conversion of a feed of 1 gmol/min of an equimolar feed of SO,CI, and inerts at 279.2 C.
Step by Step Solution
3.42 Rating (165 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemical Principles
Authors: Steven S. Zumdahl, Donald J. DeCoste
7th edition
9781133109235, 1111580650, 978-1111580650
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



