1. What is the proper balance for pharmaceutical companies between delivering the fiduciary obligation of earning a profit for owners and providing lifesaving or life-extending
1. What is the proper balance for pharmaceutical companies between delivering the fiduciary obligation of earning a profit for owners and providing lifesaving or life-extending drugs to customers? How much profit is too much profit and who determines the amount? How does that balance get achieved?
2. Should the United States consider other methods for controlling drug pricing, such as those used in some European countries? Are there other ways the United States might use market forces or incentives from government programs to control drug prices? Given that one of the most prevalent and persuasive arguments for relatively high drug prices is the high cost associated with research and development and regulatory compliance, is there a way to combat those costs?
3. What are your views on the role of patents in prescription medication? What is the proper balance of patent protection for costly research and development versus lack of competition?
4. What should be done on the issue of orphan drugs to combat high costs without viable alternatives? Should there be cost restrictions? Should there be patent restrictions?
5. What should be done in cases like Turing and Valeant Pharmaceuticals, where decades-old medications that do not have competitors are purchased and prices are raised exponentially? If you think restrictions should be imposed, what is the justification for treating that case differently than the case where a drug, with patent protection, comes to market and is priced for hundreds or thousands of dollars?
6. How can the United States and other developed countries stimulate greater research and development of treatments for NTDs and offer those drugs at prices that are affordable?
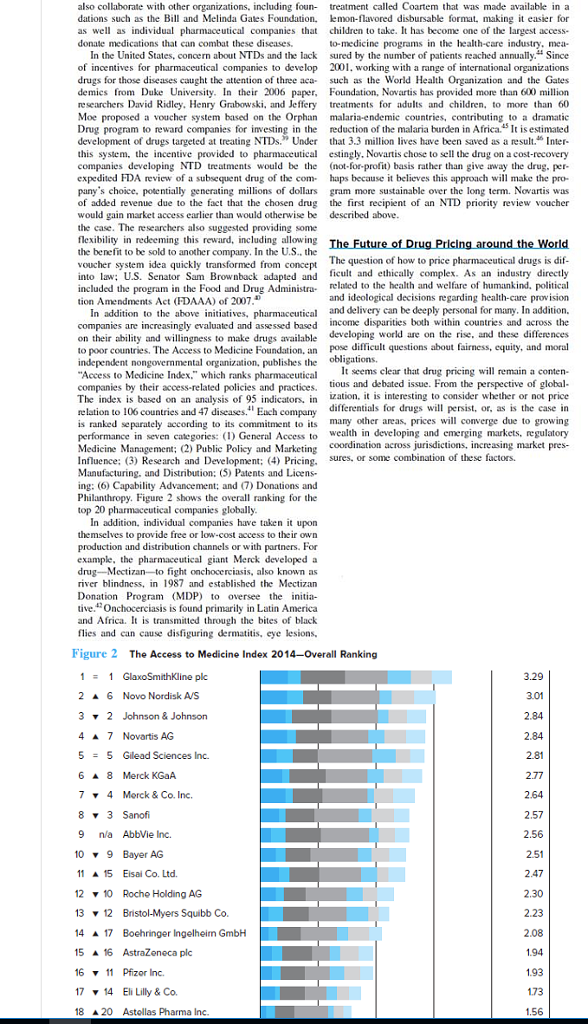
also collaborate with other organizations, including foun- dations such as the Bill and Melinda Gates Foundation, as well as individual pharmaceutical companies that donate medications that can combat these diseases. In the United States, concern about NTDs and the lack of incentives for pharmaceutical companies to develop drugs for those diseases caught the attention of three aca- demics from Duke University. In their 2006 paper, researchers David Ridley, Henry Grabowski, and Jeffery Moe proposed a voucher system based on the Orphan Drug program to reward companies for investing in the development of drugs targeted at treating NTDs." Under this system, the incentive provided to pharmaceutical companies developing NTD treatments would be the expedited FDA review of a subsequent drug of the com- pany's choice, potentially generating millions of dollars of added revenue due to the fact that the chosen drug would gain market access earlier than would otherwise be the case. The researchers also suggested providing some flexibility in redeeming this reward, including allowing the benefit to be sold to another company. In the U.S.. the voucher system idea quickly transformed from concept into law; U.S. Senator Sam Brownback adapted and included the program in the Food and Drug Administra tion Amendments Act (FDAAA) of 2007. addition to the above initiatives, pharmaceutical companies are increasingly evaluated and assessed based on their ability and willingness to make drugs available to poor countries. The Access to Medicine Foundation, an independent nongovernmental organization, publishes the "Access to Medicine Index," which ranks pharmaceutical companies by their access-related policies and practices. The index is based on an analysis of 95 indicators, in relation to 106 countries and 47 diseases." Each company is ranked separately according to its commitment to its performance in seven categories: (1) General Access to Medicine Management; (2) Public Policy and Marketing Influence; (3) Research and Development; (4) Pricing. Manufacturing, and Distribution; (5) Patents and Licens- ing: (6) Capability Advancement; and (7) Donations and Philanthropy. Figure 2 shows the overall ranking for the top 20 pharmaceutical companies globally. 1 = 1 GlaxoSmithKline plc 2 A 6 Novo Nordisk A/S 3 2 Johnson & Johnson 4A 7 Novartis AG 5 = 5 Gilead Sciences Inc. 6 A 8 Merck KGaA 7 4 Merck & Co. Inc. 83 Sanofi 9 n/a AbbVie Inc. 10 9 Bayer AG 11 15 Eisai Co. Ltd. treatment called Coartem that was made available in a lemon-flavored disbursable format, making it easier for children to take. It has become one of the largest access- to-medicine programs in the health-care industry, mea- sured by the number of patients reached annually." Since 2001, working with a range of international organizations such as the World Health Organization and the Gates Foundation, Novartis has provided more than 600 million treatments for adults and children, to more than 60 malaria-endemic countries, contributing to a dramatic reduction of the malaria burden in Africa. It is estimated that 3.3 million lives have been saved as a result." Inter- estingly, Novartis chose to sell the drug on a cost-recovery (not-for-profit) basis rather than give away the drug, per- haps because it believes this approach will make the pro- gram more sustainable over the long term. Novartis was the first recipient of an NTD priority review voucher described above. In addition, individual companies have taken it upon themselves to provide free or low-cost access to their own production and distribution channels or with partners. For example, the pharmaceutical giant Merck developed a drug-Mectizan to fight onchocerciasis, also known as river blindness, in 1987 and established the Mectizan Donation Program (MDP) to oversee the initia- tive. Onchocerciasis is found primarily in Latin America and Africa. It is transmitted through the bites of black flies and can cause disfiguring dermatitis, eye lesions, Figure 2 The Access to Medicine Index 2014-Overall Ranking 12 10 Roche Holding AG 13 12 Bristol-Myers Squibb Co. 14 17 Boehringer Ingelheimn GmbH 15 16 AstraZeneca plc 16 11 Pfizor Inc. 17 14 Eli Lilly & Co. 1820 Astellas Pharma Inc. The Future of Drug Pricing around the World The question of how to price pharmaceutical drugs is dif- ficult and ethically complex. As an industry directly related to the health and welfare of humankind, political and ideological decisions regarding health-care provision and delivery can be deeply personal for many. In addition, income disparities both within countries and across the developing world are on the rise, and these differences pose difficult questions about fairness, equity, and moral obligations. It seems clear that drug pricing will remain a conten- tious and debated issue. From the perspective of global- ization, it is interesting to consider whether or not price. differentials for drugs will persist, or, as is the case in many other areas, prices will converge due to growing wealth in developing and emerging markets, regulatory coordination across jurisdictions, increasing market pres- sures, or some combination of these factors. 3.29 3.01 2.84 2.84 2.81 2.77 2.64 2.57 2.56 2.51 2.47 2.30 2.23 2.08 1.94 1.93 1.73 1.56
Step by Step Solution
3.48 Rating (151 Votes )
There are 3 Steps involved in it
Step: 1
1 2 3 4 5 6 It is quite difficult and complex to find out the proper balance for pharmaceutical comp...
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started