Answered step by step
Verified Expert Solution
Question
1 Approved Answer
2. (11 marks total) Ethanol (CHOH) burns in a domestic furnace at a pressure of 101.3 kPa according to the following stoichiometric equation: CHOH
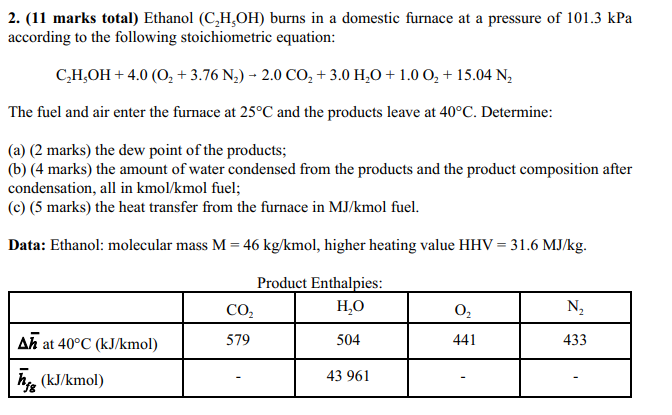
2. (11 marks total) Ethanol (CHOH) burns in a domestic furnace at a pressure of 101.3 kPa according to the following stoichiometric equation: CHOH + 4.0 (O+3.76 N) 2.0 CO+ 3.0 HO + 1.0 O + 15.04 N The fuel and air enter the furnace at 25C and the products leave at 40C. Determine: (a) (2 marks) the dew point of the products; (b) (4 marks) the amount of water condensed from the products and the product composition after condensation, all in kmol/kmol fuel; (c) (5 marks) the heat transfer from the furnace in MJ/kmol fuel. Data: Ethanol: molecular mass M = 46 kg/kmol, higher heating value HHV = 31.6 MJ/kg. Product Enthalpies: CO HO O N Ah at 40C (kJ/kmol) 579 504 441 433 hfg (kJ/kmol) 43 961
Step by Step Solution
★★★★★
3.39 Rating (155 Votes )
There are 3 Steps involved in it
Step: 1
a To determine the dew point of the products we need to compare the partial pressure of water vapor in the products with the saturation pressure of wa...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


