Answered step by step
Verified Expert Solution
Question
1 Approved Answer
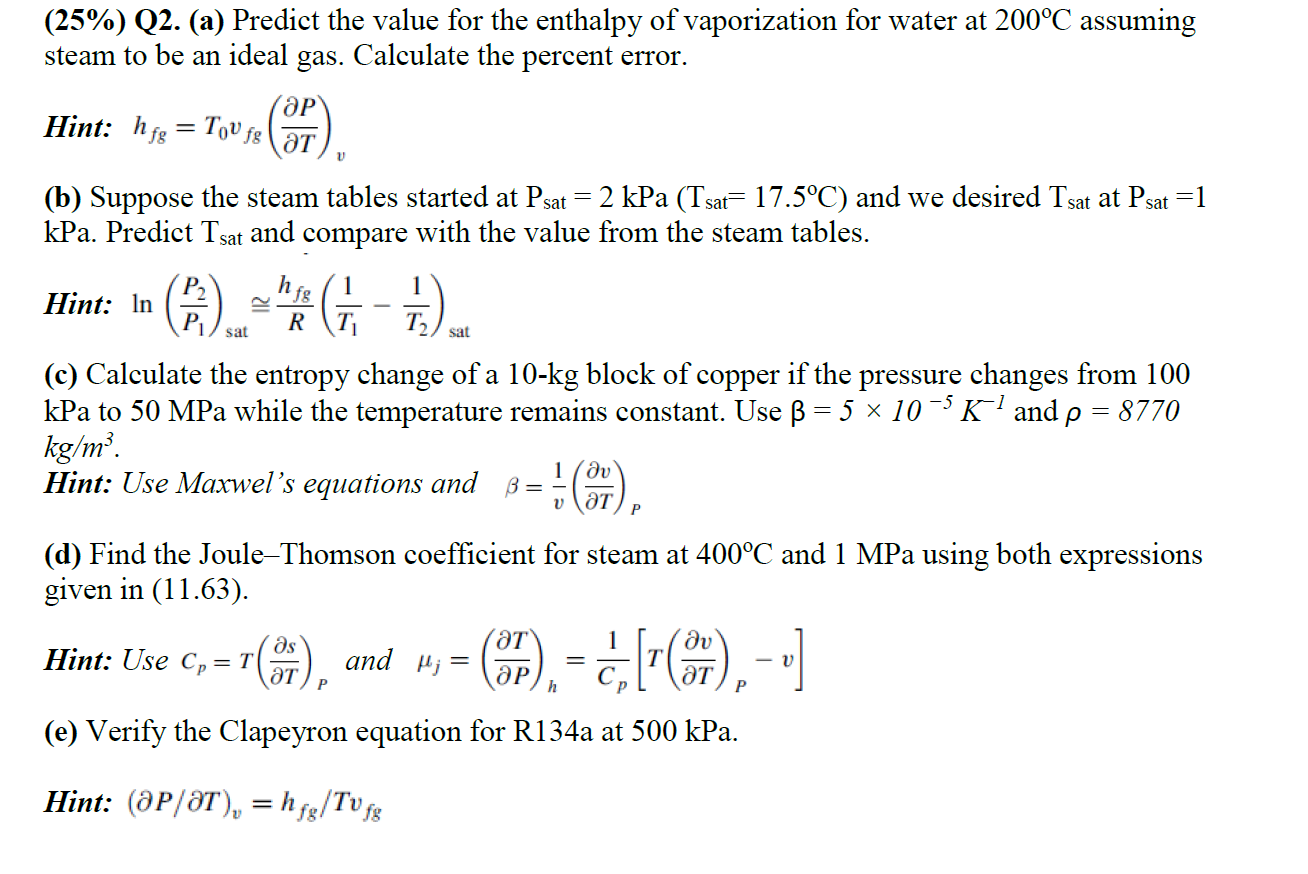
( 2 5 % ) Q 2 . ( a ) Predict the value for the enthalpy of vaporization for water at 2 0 0
Qa Predict the value for the enthalpy of vaporization for water at assuming Qa Predict the value for the enthalpy of vaporization for water at assuming
steam to be an ideal gas. Calculate the percent error.
Hint:
b Suppose the steam tables started at kPa and we desired at
kPa. Predict and compare with the value from the steam tables.
Hint: ~
c Calculate the entropy change of a kg block of copper if the pressure changes from
kPa to MPa while the temperature remains constant. Use and
Hint: Use Maxwel's equations and
d Find the JouleThomson coefficient for steam at and MPa using both expressions
given in
Hint: Use and
e Verify the Clapeyron equation for Ra at kPa.
Hint: elT
steam to be an ideal gas. Calculate the percent error.
Hint:
b Suppose the steam tables started at kPa and we desired at
kPa. Predict and compare with the value from the steam tables.
Hint: ~
c Calculate the entropy change of a kg block of copper if the pressure changes from
kPa to MPa while the temperature remains constant. Use and
Hint: Use Maxwel's equations and
d Find the JouleThomson coefficient for steam at and MPa using both expressions
given in
Hint: Use and
e Verify the Clapeyron equation for Ra at kPa.
Hint: elT
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


