Answered step by step
Verified Expert Solution
Question
1 Approved Answer
2. A curve fit for the enthalpy of molecular oxygen is given as: h(T) = 259.8(3.782T2.99710-32 +9.84710-673 -9 -9.681x10- +3.24310-12 1.064103) J/kg T5 5
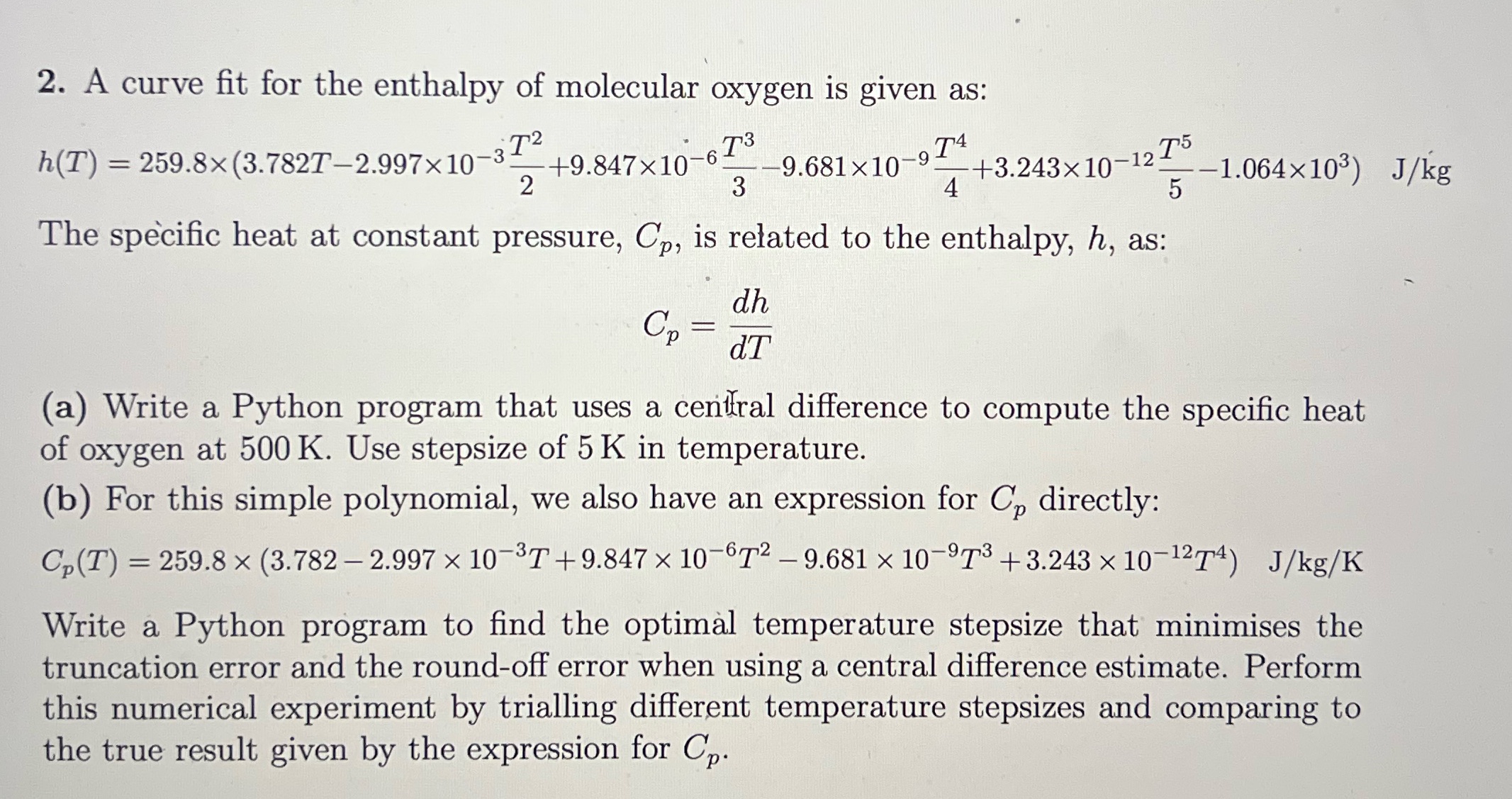
2. A curve fit for the enthalpy of molecular oxygen is given as: h(T) = 259.8(3.782T2.99710-32 +9.84710-673 -9 -9.681x10- +3.24310-12 1.064103) J/kg T5 5 2 3 T4 4 The specific heat at constant pressure, Cp, is related to the enthalpy, h, as: Cp = = dh dT (a) Write a Python program that uses a central difference to compute the specific heat of oxygen at 500 K. Use stepsize of 5 K in temperature. (b) For this simple polynomial, we also have an expression for C, directly: C,(T) = 259.8 (3.782-2.997 x 10-37 +9.847 10-672-9.681 10-973 +3.243 10-1274) J/kg/K Write a Python program to find the optimal temperature stepsize that minimises the truncation error and the round-off error when using a central difference estimate. Perform this numerical experiment by trialling different temperature stepsizes and comparing to the true result given by the expression for Cp.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


