Answered step by step
Verified Expert Solution
Question
1 Approved Answer
2. A simplified phase diagram of alkali feldspar system is shown below. The system shows a miscibility gap in solid-solution of solid at low temperatures.
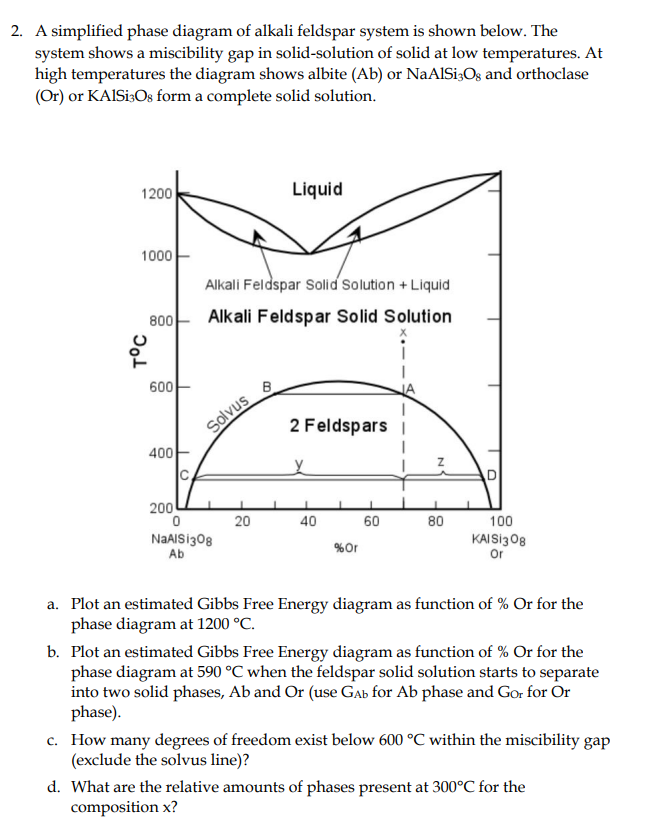 2. A simplified phase diagram of alkali feldspar system is shown below. The system shows a miscibility gap in solid-solution of solid at low temperatures. At high temperatures the diagram shows albite (Ab) or NaAlSi3O8 and orthoclase (Or) or KAlSi3O8 form a complete solid solution. a. Plot an estimated Gibbs Free Energy diagram as function of % Or for the phase diagram at 1200C. b. Plot an estimated Gibbs Free Energy diagram as function of \% Or for the phase diagram at 590C when the feldspar solid solution starts to separate into two solid phases, Ab and Or (use GAb for Ab phase and GOr for Or phase). c. How many degrees of freedom exist below 600C within the miscibility gap (exclude the solvus line)? d. What are the relative amounts of phases present at 300C for the composition x
2. A simplified phase diagram of alkali feldspar system is shown below. The system shows a miscibility gap in solid-solution of solid at low temperatures. At high temperatures the diagram shows albite (Ab) or NaAlSi3O8 and orthoclase (Or) or KAlSi3O8 form a complete solid solution. a. Plot an estimated Gibbs Free Energy diagram as function of % Or for the phase diagram at 1200C. b. Plot an estimated Gibbs Free Energy diagram as function of \% Or for the phase diagram at 590C when the feldspar solid solution starts to separate into two solid phases, Ab and Or (use GAb for Ab phase and GOr for Or phase). c. How many degrees of freedom exist below 600C within the miscibility gap (exclude the solvus line)? d. What are the relative amounts of phases present at 300C for the composition x Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


