Answered step by step
Verified Expert Solution
Question
1 Approved Answer
2) An open Energy-Interaction diagram. Refer to the Diagrammatic Representation in the Energy- Interaction Model foldout. Don't forget to include algebraic expressions of energy
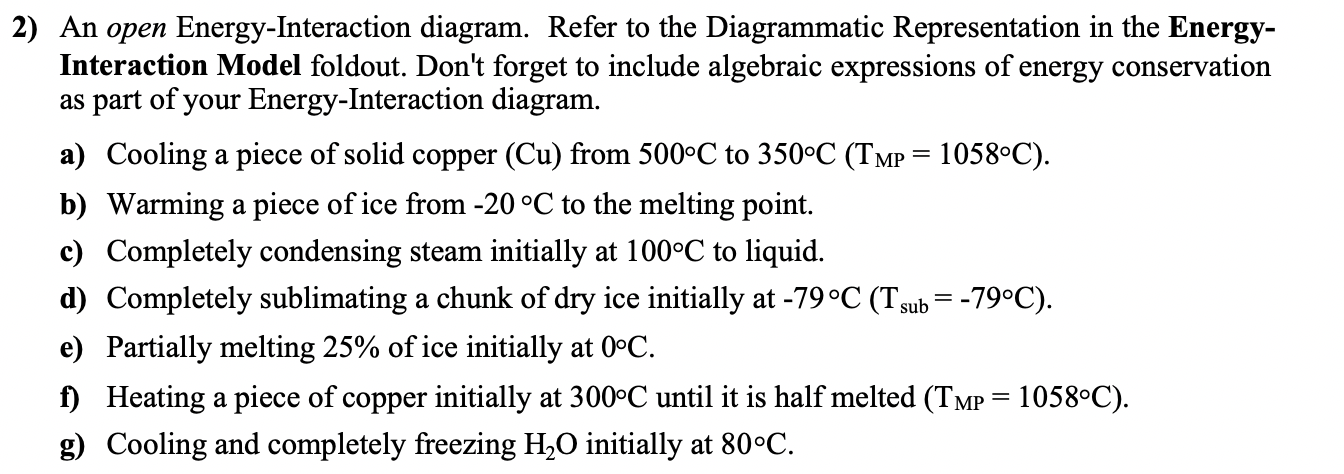
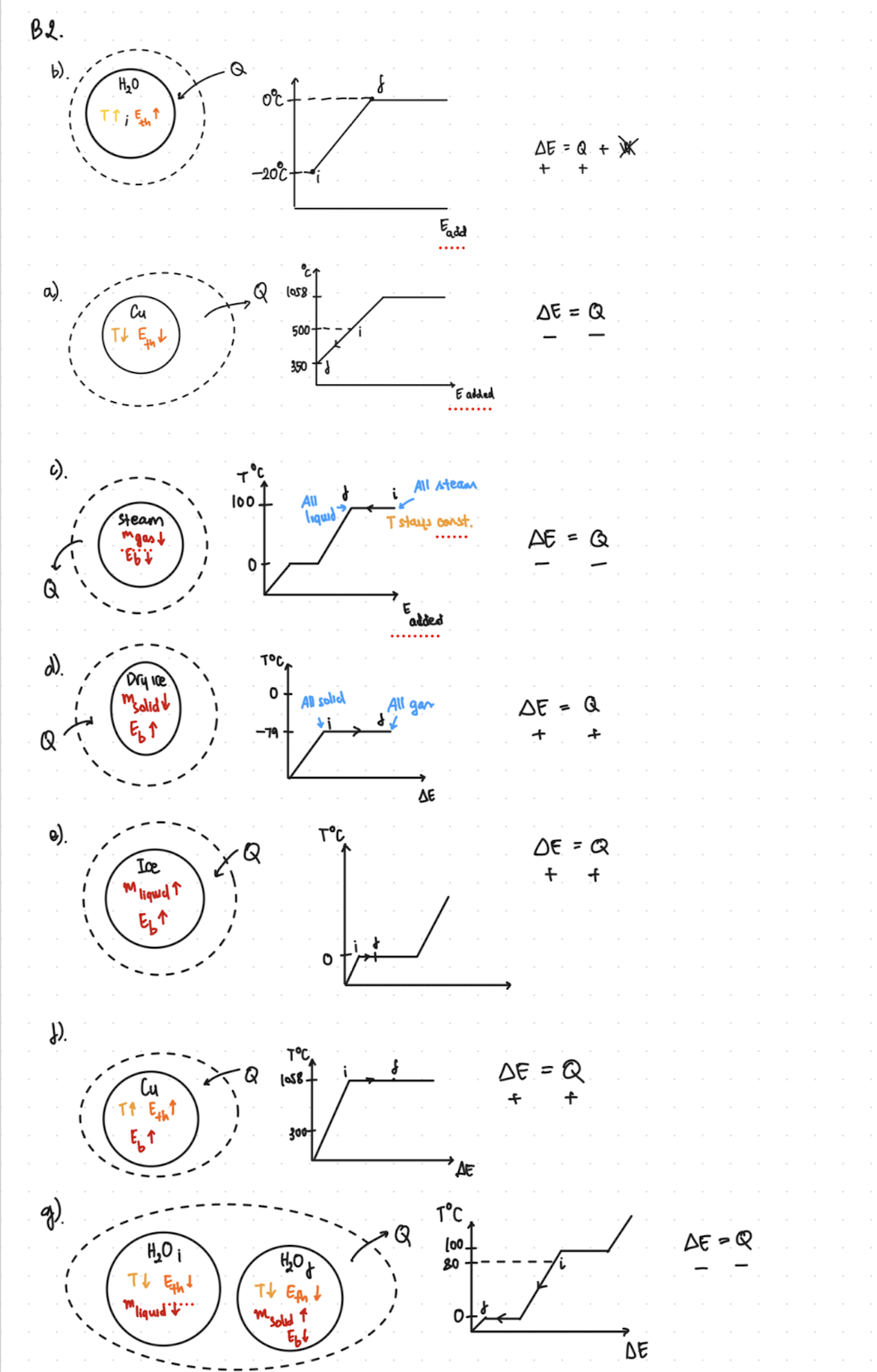
2) An open Energy-Interaction diagram. Refer to the Diagrammatic Representation in the Energy- Interaction Model foldout. Don't forget to include algebraic expressions of energy conservation as part of your Energy-Interaction diagram. a) Cooling a piece of solid copper (Cu) from 500C to 350C (TMP = 1058C). b) Warming a piece of ice from -20 C to the melting point. c) Completely condensing steam initially at 100C to liquid. d) Completely sublimating a chunk of dry ice initially at -79 C (Tsub = -79C). e) Partially melting 25% of ice initially at 0C. f) Heating a piece of copper initially at 300C until it is half melted (TMP = 1058C). g) Cooling and completely freezing HO initially at 80C. B2. b). HO Q -20C Fadd 1058. 500- 350 E added DE = Q + K ++ DE = Q All steam 100 i Steam mgast Eb7 All liquid T stays const. AE = Q 0 added Dry Ice msolid 0 All solid All gar = Q E +i -79+ + 6 4 + M Ice "liquid TC DE = Q + + TC 1058+ DE = Q T Eth + ET 300 HOi Tt Eth mliquid t... HO m solid Ebb TC Q (00 80 DE=Q
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


