Answered step by step
Verified Expert Solution
Question
1 Approved Answer
2 and 3 You purchase six oranges that weigh a total of 3 (by and 3.7 ounces. After cutting them open and squeezing all the
2 and 3 
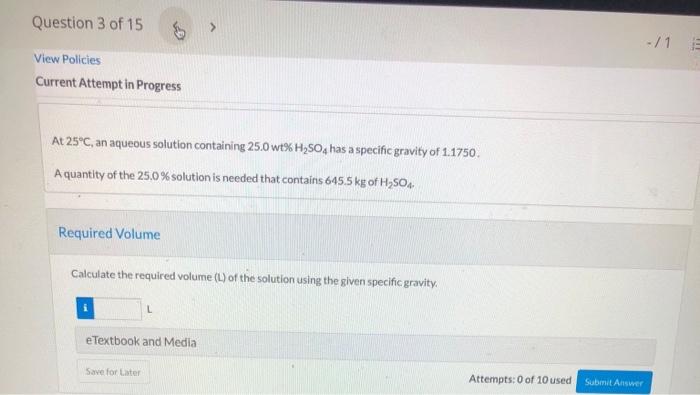
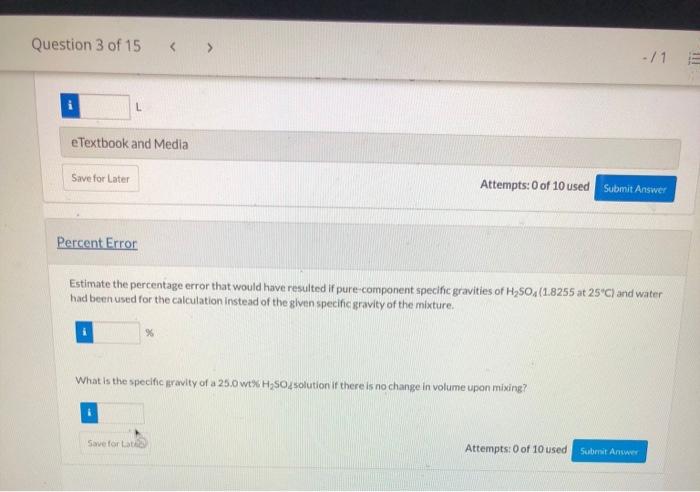
You purchase six oranges that weigh a total of 3 (by and 3.7 ounces. After cutting them open and squeezing all the juice your strength allows into a large measuring cup, you weigh the remaining pulp and orange peels. They weigh 1 lband 15.7 ounces and the total volume of the juice is 2.22 cups. What is the specific gravity of the orange juice that you squeezed? Question 3 of 15 View Policies Current Attempt in Progress At 25C, an aqueous solution containing 25.0 wt% H250, has a specific gravity of 1.1750 A quantity of the 25.0 % solution is needed that contains 645.5 kg of HSO4 Required Volume Calculate the required volume (L) of the solution using the given specific gravity. L e Textbook and Media Save for Later Attempts: 0 of 10 used Submit Answer Question 3 of 15 -/1 11 e Textbook and Media Save for Later Attempts: 0 of 10 used Submit Answer Percent Error Estimate the percentage error that would have resulted if pure-component specific gravities of H250 (1.8255 at 25C) and water had been used for the calculation instead of the given specific gravity of the mixture, What is the specific gravity of a 25.0 wt%H SO solution if there is no change in volume upon mixing? Save for Late Attempts: 0 of 10 used Submit 


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


