Question
2) (application) Consider the processes (A)-(D), as plotted on a PV diagram. Each of these processes takes the same ideal gas from an identical
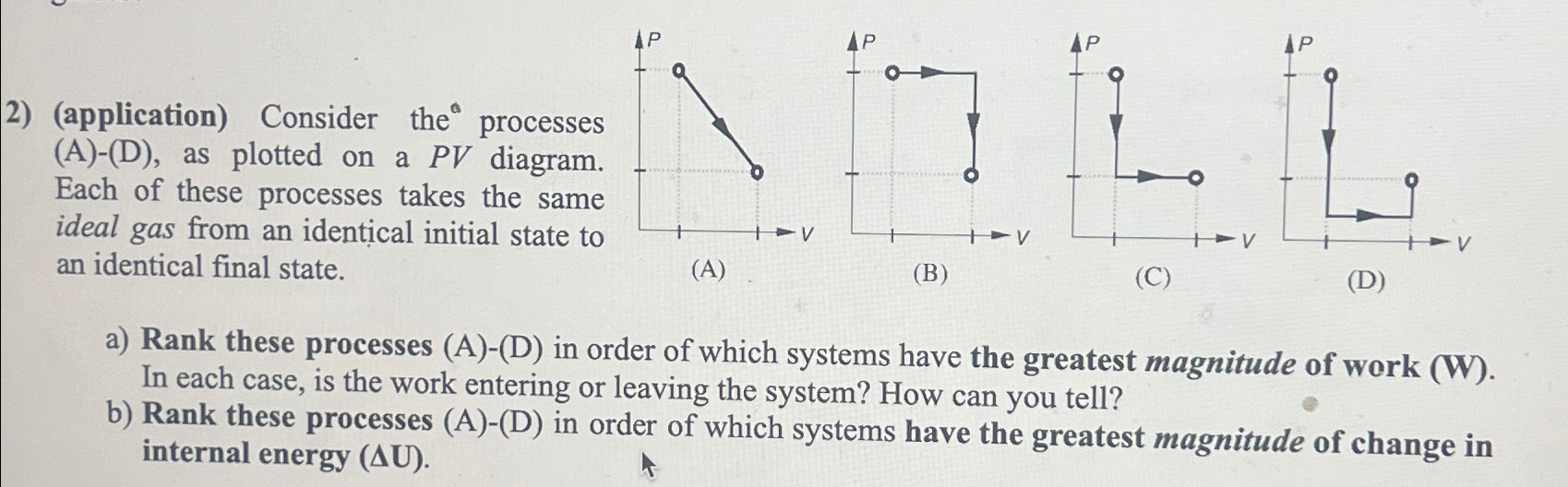
2) (application) Consider the processes (A)-(D), as plotted on a PV diagram. Each of these processes takes the same ideal gas from an identical initial state to an identical final state. LOLL (A) (B) (C) (D) a) Rank these processes (A)-(D) in order of which systems have the greatest magnitude of work (W). In each case, is the work entering or leaving the system? How can you tell? b) Rank these processes (A)-(D) in order of which systems have the greatest magnitude of change in internal energy (AU).
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Cornerstones Of Cost Management
Authors: Don R. Hansen, Maryanne M. Mowen
3rd Edition
9781305147102, 1285751787, 1305147103, 978-1285751788
Students also viewed these Physics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



