Answered step by step
Verified Expert Solution
Question
1 Approved Answer
2. As a result of Cthulhu's body heat, the column of ocean water above him has roughly uniform temperature across all depths. According to
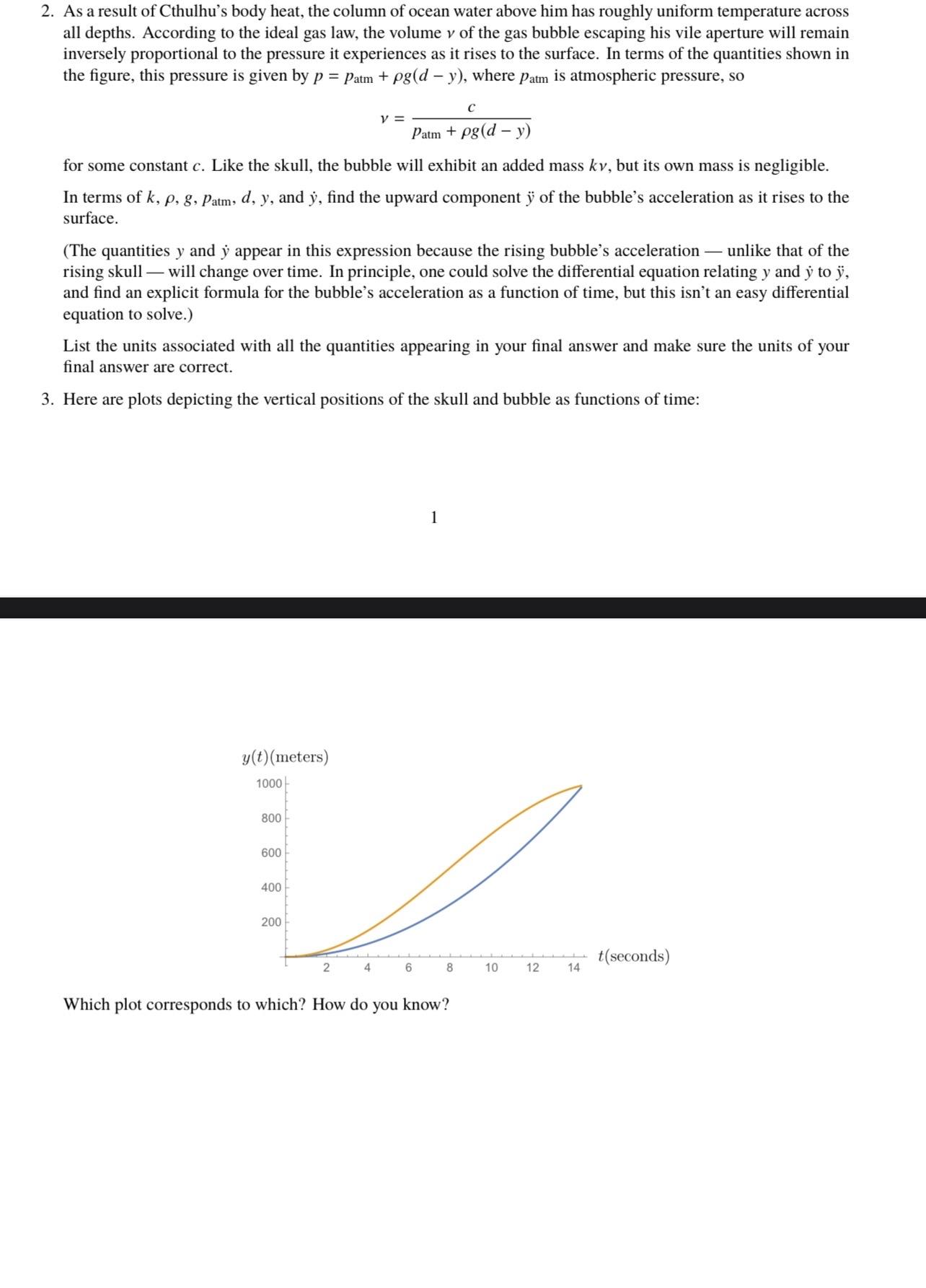
2. As a result of Cthulhu's body heat, the column of ocean water above him has roughly uniform temperature across all depths. According to the ideal gas law, the volume v of the gas bubble escaping his vile aperture will remain inversely proportional to the pressure it experiences as it rises to the surface. In terms of the quantities shown in the figure, this pressure is given by p = Patm + pg(d - y), where patm is atmospheric pressure, so for some constant c. Like the skull, the bubble will exhibit an added mass kv, but its own mass is negligible. In terms of k, p, g, Patm, d, y, and y, find the upward component y of the bubble's acceleration as it rises to the surface. (The quantities y and y appear in this expression because the rising bubble's acceleration - unlike that of the rising skull- 1 will change over time. In principle, one could solve the differential equation relating y and y to y, and find an explicit formula for the bubble's acceleration as a function of time, but this isn't an easy differential equation to solve.) y(t) (meters) 1000- List the units associated with all the quantities appearing in your final answer and make sure the units of your final answer are correct. 3. Here are plots depicting the vertical positions of the skull and bubble as functions of time: 800 600 400 y = 200 C Patm + pg (d - y) 2 4 6 1 8 Which plot corresponds to which? How do you know? 10 12 14 t(seconds)
Step by Step Solution
★★★★★
3.58 Rating (158 Votes )
There are 3 Steps involved in it
Step: 1
To determine the upward acceleration component of the bubble as it rises to the surface we first need to establish the equation of motion for the bubb...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


