Answered step by step
Verified Expert Solution
Question
1 Approved Answer
2. Calculate the stoichiometric air-fuel ratio for the combustion of a sample of dry anthracite of the following composition by mass: C = 90%;
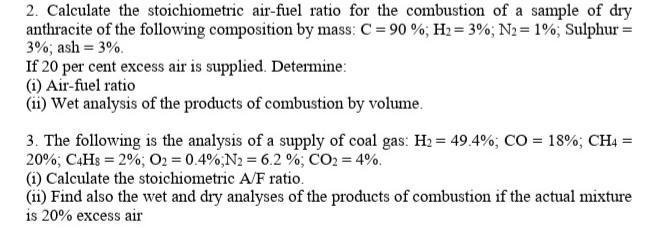
2. Calculate the stoichiometric air-fuel ratio for the combustion of a sample of dry anthracite of the following composition by mass: C = 90%; H2=3%; N2 = 1%; Sulphur = 3%; ash = 3%. If 20 per cent excess air is supplied. Determine: (i) Air-fuel ratio (ii) Wet analysis of the products of combustion by volume. 3. The following is the analysis of a supply of coal gas: H = 49.4%; CO = 18% ; CH4 = 20%; C4H8=2%; O2 = 0.4%; N2 = 6.2%; CO2 = 4%. (i) Calculate the stoichiometric A/F ratio. (ii) Find also the wet and dry analyses of the products of combustion if the actual mixture is 20% excess air
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


