Question
2) Carbon dioxide at 25 C, 1 atm enters a reactor operating at steady state and dissociates, giving an equilibrium mixture of CO2, CO,
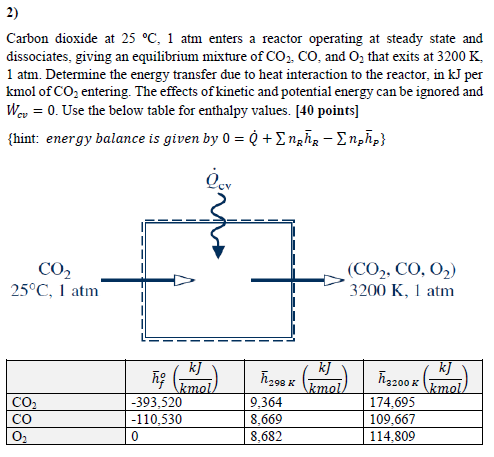
2) Carbon dioxide at 25 C, 1 atm enters a reactor operating at steady state and dissociates, giving an equilibrium mixture of CO2, CO, and O that exits at 3200 K 1 atm. Determine the energy transfer due to heat interaction to the reactor, in kJ per kmol of CO2 entering. The effects of kinetic and potential energy can be ignored and We 0. Use the below table for enthalpy values. [40 points] - {hint: energy balance is given by 0 = Q + n nphp} Qev CO 25C, 1 atm CO CO _ (CO2, CO, O,) 3200 K, 1 atm h? k] Akmol 1298x (+1) kJ F3200 x (D) kJ mol) -393,520 9.364 174,695 -110,530 8,669 109,667 0 8,682 114,809
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Thermodynamics An Interactive Approach
Authors: Subrata Bhattacharjee
1st edition
130351172, 978-0130351173
Students also viewed these Mechanical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



