Answered step by step
Verified Expert Solution
Question
1 Approved Answer
2. Compare the three values you obtained for the concentration of citric acid (g/L). Did your three titrations yield consistent results? If the values are
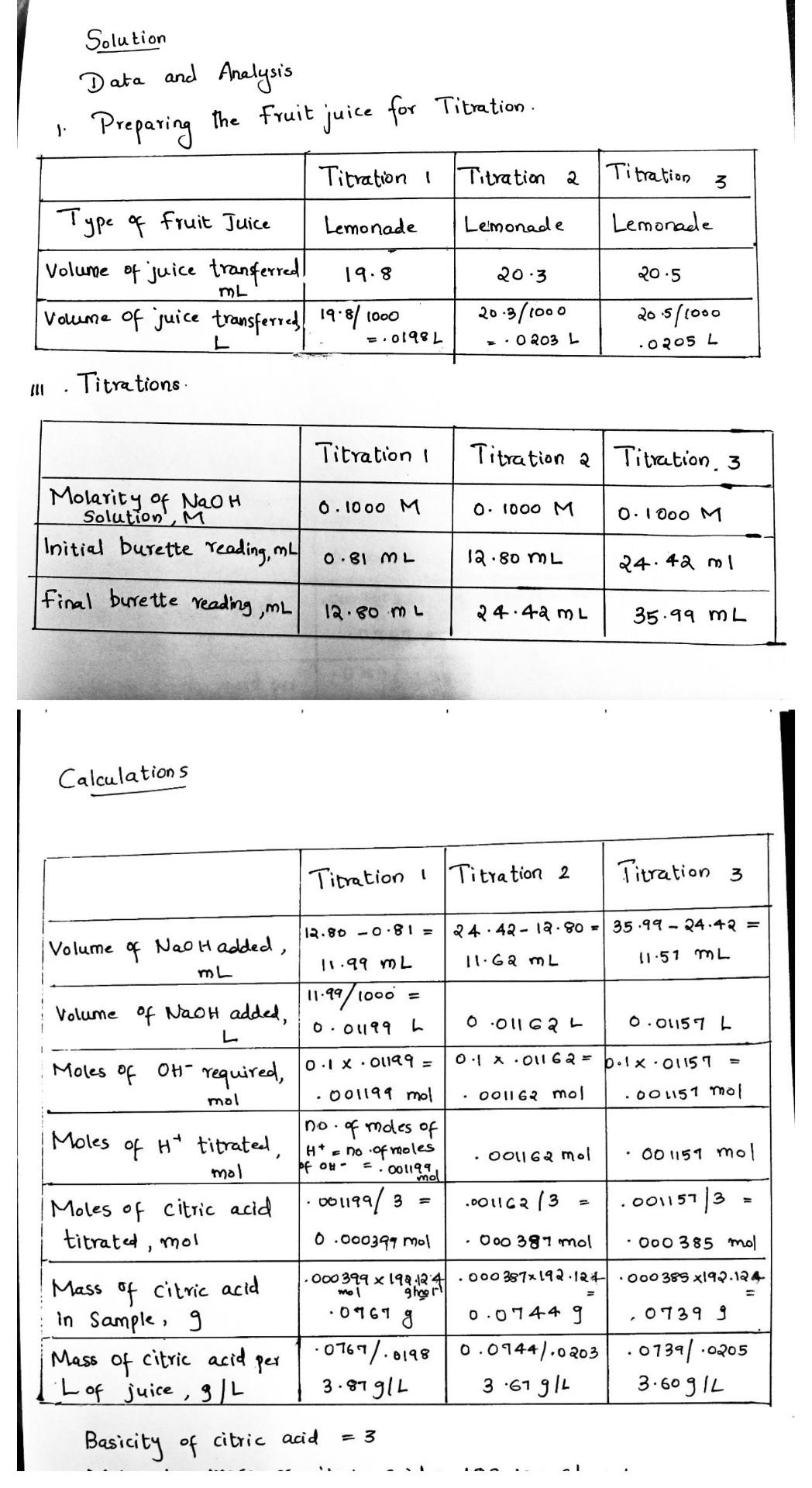
2. Compare the three values you obtained for the concentration of citric acid (g/L). Did your three titrations yield consistent results? If the values are not exactly the same, why do you suppose one might be higher or lower than the others? 3. Compare your results with those of other class members who analyzed different juices. List the juices in order of increasing acidity: Type of Juice Citric acid concentration, (g/L) Student(s) from whom you obtained the data 4. What problem would occur if you tried to use this same procedure to determine the acidity of tomato juice? What change would you make to the procedure? 1: Solution Data and Analysis Preparing the fruit juice for Titration . Titration I Type of fruit Juice Lemonade Lemonade Volume of juice transferred! volume of juice transferred 1918/1 Titration a | Titration 3 Lemonade 19.8 20:3 20.5 ML t000 =.01981 20-3/1000 -.0 203 L 20.5/1000 L .52 Titrations Titration I Titration a Titration 3 0.1000 M 0. 1000 M 0.1000 M Molarity of NaOH Solution, M Initial burette reading, my 0.81 ML 12.80 mL 24.42 ml final burette reading mL 12.80 mL 24.44 m2 35.99 ML Calculations Titration Titration 2 Titration 3 11.51 mL = Moles of . . 12.80 -0.81 = 24.42 - 12.80 = 35.99-24.42 = |Volume of Nao Hadded, 11.99 mL 11.G2 mL mL 11.99/1000 = Volume of Naot added L 0.01199 0.01162 L 0.01157L OX.01162 = b.lx. 01157 0.1 x .00199 = OH required, mol .001199 mol 001162 mol .00 1151 mol no of moles of Moles of H" titrated, Hno .of moles 001162 mol 001151 mol mol be ou- mol Moles of citric acid 001199/3 .001162/3 .00115713 titrated, mol 0.000397 mol 000 391 mol .000 385 mol .000 399 x 19:12:41 .000 387x192.124 .000 385 x192.124 Mass of citric acid ghoor! in Sample, 9 0961 g 0.0744 9 07399 Mass of citric acid per -0767/.0198 0.0944/.0203 .0739/-0205 L of juice, g/L 3.87 g/L 3.67 914 3.609 L Ooti, = 2 . = 3 Basicity of citric acid
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


